The behaviour of each basal cell carcinoma is known to be different according to the histological growth pattern. Among these aggressive lesions, sclerodermiform basal cell carcinomas are the most common type. This is a challenging-to-treat lesion due to its deep tissue invasion, rapid growth, risk of metastasis and overall poor prognosis if not diagnosed in early stages.
ObjectiveTo investigate if sclerodermiform basal cell carcinomas are diagnosed later compared to non-sclerodermiform basal cell carcinoma Method: All lesions excised from 2000 to 2010 were included. A pathologist classified the lesions in two cohorts: one with specimens of non-aggressive basal cell carcinoma (superficial, nodular and pigmented), and other with sclerodermiform basal cell carcinoma. For each lesion, we collected patient’s information from digital medical records regarding: gender, age when first attending the clinic and the tumor location.
Results:1256 lesions were included, out of which 296 (23.6%) corresponded to sclerodermiform basal cell carcinoma, whereas 960 (76.4%) were non-aggressive subtypes of basal cell carcinoma. The age of diagnosis was: 72.78±12.31 years for sclerodermiform basal cell and 69.26±13.87 years for non-aggressive basal cell carcinoma (P<.0001). Sclerodermiform basal cell carcinomas are diagnosed on average 3.52 years later than non-aggressive basal cell carcinomas. Sclerodermiform basal cell carcinomas were diagnosed 3.40 years and 2.34 years later than non-aggressive basal cell carcinomas in younger and older patients respectively (P=.002 and P=.03, respectively).
Study Limitations:retrospective design.
Conclusion:The diagnostic accuracy and primary clinic conjecture of sclerodermiform basal cell carcinomas is quite low compared to other forms of basal cell carcinoma such as nodular, superficial and pigmented. The dermoscopic vascular patterns, which is the basis for the diagnosis of non-melanocytic nonpigmented skin tumors, may not be particularly useful in identifying sclerodermiform basal cell carcinomas in early stages. As a distinct entity, sclerodermiform basal cell carcinomas show a lack of early diagnosis compared to less-aggressive subtypes of BCC, and thus, more accurate diagnostic tools apart from dermatoscopy are required to reach the goal of early-stage diagnosis of sclerodermiform basal cell carcinomas.
The current classification of basal cell carcinoma (BCC) establishes four main clinical variants of this tumour: superficial, nodular, infiltrative and pigmented BCC.1 The behaviour of each one is known to be different according to the histological growth pattern. Histologically aggressive BCCs are not uncommon according to large studies from referral centers’ researches, which report incidences ranging from 2.5 to 44%.2-6 Certain behavioural and/or environmental factors, apart from UV radiation, such as kidney transplant recipients have been associated with a higher incidence of aggressive subtypes of BCC.7,8 Among these aggressive lesions, sclerodermiform basal cell carcinomas (SBCC) are the most common type. This is a challenging-to-treat lesion due to its deep tissue invasion, rapid growth, risk of metastasis and overall poor prognosis if treatment is not initiated in early stages. Even after surgery, considerable recurrence rates (up to 40%) may occur, while SBCC respond poorly to non-surgical modalities.9-11 An early diagnosis of SBCC is the primary method to establish an effective secondary prevention measure to decrease disfiguring surgical excisions and concomitant comorbidity.
The primary diagnostic method of this condition is clinical examination with the use of dermoscopy, whereas the pathological evaluation is confirmatory. Despite the aggressiveness and the impact on the quality of life, very limited data are available focused on the diagnosis of this condition.
The main objective of this work is to investigate if SBCC are diagnosed as early as non-sclerodermiform basal cell carcinoma. A second aim is to analyze if there is any difference at the age of diagnosis regarding gender.
MethodAfter having the study protocol approved by the Ethics Committee from our center, we designed a retrospective study and all the lesions of BCC excised in our hospital from 2000 to 2010 were included in the study. A pathologist classified the lesions according to the histological pattern in two cohorts: one with specimens of non-aggressive BCC (superficial, nodular and pigmented), and other with sclerodermiform BCC. Although there are several ways of classify a BCC as aggressive, we just focused only on sclerodermiform type._ Only cases having an excision with margins free of disease were included, and histopathologic examinations as result of a punch or shave biopsy were excluded. Subjects with previous history of squamous cutaneous carcinoma and other types of BCC such as micronodular were also dismissed. For each lesion, we collected patients’ information from the digital medical records regarding: gender, age when first attended to the clinic and tumor location.
The first outcome was the age of patients when the diagnosis was made in each cohort. Differences in the age at diagnosis regarding gender and longevity were also analyzed.
To examine whether there was a significant statistical difference in the mean age of diagnosis between the two cohorts, Student T test was used (with their 95% confidence intervals) with the SPSS version 20 software. We stratified the sample in two ways: a) by decades and b) by longevity of the patients, to take into account possible confoundings. All p values were 2-sided and p values less than .05 were considered statistically significant.
ResultsOne thousand, two hundred and fifty-six cases of BBC were identified, out of which 296 (23.6%) corresponded to sclerodermiform BCC, whereas 960 (76.4%) were non-aggressive subtypes of BCC. One hundred and twenty-six cases of BCC were excluded since they did not match the inclusion criteria. The age at diagnosis was: 72.78±12.31 years for SBCC and 69.26±13.87 years for non-aggressive BCC (P<.0001). SBCC were diagnosed on average 3.52 years later than non-aggressive BCCs.
The most frequent location was the nose (257 lesions, 20.5%) followed by: cheek (203 lesions, 16.2%), trunk (191 lesions, 15.2%), forehead (144 lesions, 11.5%), periocular (133 lesions, 10.6%), periauricular (133 lesions, 10.6%), scalp (73 lesions, 5.8%), neck (47 lesions, 3.7%), limbs (44 lesions, 3.5%) and perioral (31 lesions, 2.5%).
When we stratified by gender, in the subgroup of women the age of diagnosis was 72.35±13.93 years for SBCC and 67.75±14.58 years for non-aggressive BCC (P=.004), whereas in the subgroup of men the age of diagnosis was 73.01±11.34 years for SBCC and 70.37±13.22 years for non-aggressive BCC (P=.015). On average, SBCC were diagnosed 4.61 years and 2.64 years later than non-aggressive BCCs in women and men respectively.
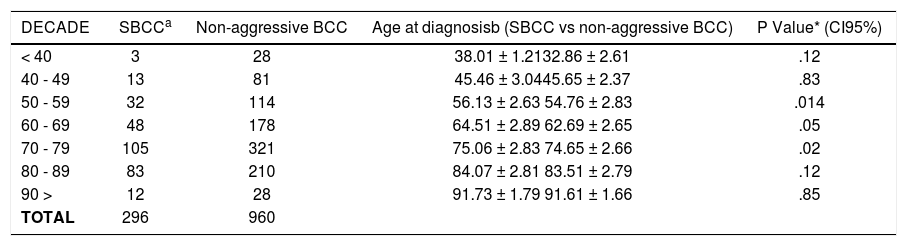
The stratified analysis by decades showed that there were statistical differences in the age at diagnosis in the 50s, 60s, and 70s. Table 1 summarizes the ages of diagnosis for SBCC and non-aggressive BCC according to each decade.
Difference in the age at diagnosis of SBCC vs non-aggressive BCC stratified by groups of age (decades)
| DECADE | SBCCa | Non-aggressive BCC | Age at diagnosisb (SBCC vs non-aggressive BCC) | P Value* (CI95%) |
|---|---|---|---|---|
| < 40 | 3 | 28 | 38.01 ± 1.2132.86 ± 2.61 | .12 |
| 40 - 49 | 13 | 81 | 45.46 ± 3.0445.65 ± 2.37 | .83 |
| 50 - 59 | 32 | 114 | 56.13 ± 2.63 54.76 ± 2.83 | .014 |
| 60 - 69 | 48 | 178 | 64.51 ± 2.89 62.69 ± 2.65 | .05 |
| 70 - 79 | 105 | 321 | 75.06 ± 2.83 74.65 ± 2.66 | .02 |
| 80 - 89 | 83 | 210 | 84.07 ± 2.81 83.51 ± 2.79 | .12 |
| 90 > | 12 | 28 | 91.73 ± 1.79 91.61 ± 1.66 | .85 |
| TOTAL | 296 | 960 |
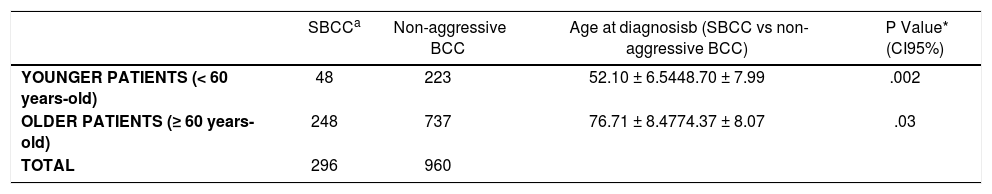
When we stratified by longevity, we observed that in the subgroup of younger patients (< 60 years-old) the age of diagnosis was 52.10±6.54 years for SBCC and 48.70 ± 7.99 years for non-aggressive BCC (P=.002), whereas in the subgroup of older patients (≥ 60 years-old) the age of diagnosis was 76.71 ± 8.47 years for SBCC and 74.37 ± 8.07 years for non-aggressive BCC (P=.03). See Table 2. Thus, on average, SBCC were diagnosed 3.40 years and 2.34 years later than non-aggressive BCCs in younger and older patients respectively.
Difference in the age at diagnosis of SBCC compared with non-aggressive BCC in younger vs older patients
Although BCCs grow slowly and rarely metastasize, there are certain subtypes which can be aggressive and disfiguring causing significant morbidity and mortality. Since there are no specific biomarker available for distinguishing aggressive forms from non-aggressive forms, having a prompt clinical conjecture is the key to lead an early diagnosis, hence it is essential as a measure of secondary prevention. In this study, we aimed to determine whether there is any difference between SBCC and non-aggressive (superficial, nodular and pigmented) BCC regarding the onset diagnosis.
Our study reveals significant differences in the age of patients at the time of diagnosis: we have observed that the onset diagnosis of SBCC is made later compared to non-aggressive BCC in all groups of the ages, and these differences were present regardless the gender and longevity of the patients.
The dermoscopic findings of basal cell carcinoma (BCC) were first described more than a decade ago and the list of BCC-related criteria has been several times updated and enriched. Today, the dermatoscope is considered the key tool for the diagnosis of BCC, since it allows its early detection and enables its differentiation from other skin tumors.12 However, most of the dermatoscopic literature on BCC to date primarily concerns pigmented variants and little is known about other morphologic variants of BCC.13
An abundant stroma is a characteristic feature of SBCC and constitutes the major part of the tumor volume. Histologically, collagen deposition is evident and more abundant than in other types of BCC.14 Thus, its clinical and dermoscopic presentations differ from the other three main clinical subtypes. SBCC has been reported to have a particular dermatoscopic pattern, consisting of shiny white-red structureless areas and arborizing vessels of a smaller caliber and less tendency to branch into finer capillaries compared to those observed in nodular BCC.15,16 Analysis of our results showed that overall SBCC are diagnosed on average 3.52 years later than non-aggressive BCCs. In our clinic, we have a digital dermatoscope_ that is commonly used by all the staff from our department in the daily practice for differential diagnosis of skin tumors, and this device was used to make the primary clinical diagnosis of all the lesions included in the study. One explanation of our findings is that the dermoscopic vascular patterns, which is the basis for the diagnosis of non-melanocytic nonpigmented skin tumors, may not be particularly useful in identifying SBCC in early stages in the same way that it is for the nodular, superficial and pigmented BCCs, since those latter subtypes were diagnosed significantly earlier in our study.16 Consistently with our findings, Popadić M conducted a recent prospective study to investigate the dermoscopic features between different histological types of BCC.13 The author concluded that the dermatoscopy is not accurate in distinguishing which BCCs are more aggressive and that the dermoscopic features in BCCs depends on the thickness of the tumor and not on its histologic nature.
The histologic explanation of our findings may be based on the growing pattern of SBCC: these lesions have a deep invasive development, and often reaching large dimensions is required before the arborizing microvessels are developed and the correct diagnosis can be made. In a recent study, analyzing the morphometric elements of the nucleus and chromatin’s homogeneity of BCC, authors found that superficial and nodular BCC had a lower chromatin intensity within the nucleus, while SBCC presented with higher morphometric rates of nucleus size. The authors suggest that these differences may be linked with the distinct clinical behaviour and growth of each lesions: the superficial and nodular BCC with prominently horizontal growth and lower degree of invasiveness, in contrast to the early and insidious invasiveness of the dermis in SBCC.17 This histological heterogeneity supports our findings of SBCC with deeper infiltration being diagnosed later than more superficial lesions, which can be detected more easily.
Based on our results, we hypothesized that both classic and non-classic BBC criteria described previously may not be enough to support the diagnosis of SBCC particularly in early lesions that may lack those BCC patterns.16,18 However, further studies are needed to examine whether the new described dermatoscopic vascular patterns can help in early diagnosis of SBCC or if new diagnostic modalities such as reflectance confocal microscopy or high-definition optical coherence tomography are required.16
One factor which has been observed associated with delaying diagnosis of BCC is the denial of illness and this is particularly relevant in SBCC, because unlike the non-aggressive basal cell carcinomas which appear mainly as a small, dense, skin coloured nodule with small visible blood vessels and central crust (nodular BCC) and in certain cases as red, sometimes scaling mark or scar (superficial BCC), SBCC appear as slow-growing, mild, skin-coloured plaque or nodule and patients interpret them as harmless changes in their skin.19,20 The denial of illness sometimes is related to the age of the patients with older patients being less careful with any skin changes than younger patients.21,22 Thus, the age might confound the later initial diagnosis for SBCC that we found in the first analysis. To control this, we stratified the subjects in our study by decades, and results were that the diagnosis of SBCC was later than in non-aggressive BCC in all decades, with significant differences at 50s, 60s, and 70s (P<.05).
In a similar way, previous studies in diagnosis of BCC have found that older patients (age >64 years) were more likely to wait to seek care than younger patients.19,22 Therefore again, the age might confound our primary outcome of later initial diagnosis for SBCC. To ensure bias does not occur, we controlled this possible confounder by analyzing separately older patients (≥ 60 years-old) and younger patients (< 60 years-old), and we observed that in both cohorts SBCC were diagnosed later than other BCCs: on average, 3.40 years and 2.34 years later in younger and older patients respectively.
After adjusting for gender, we obtained the same overall outcome: SBCC were significantly diagnosed later than non-aggressive BCC in both women and men.
To our best knowledge, this is a novel study to address the initial diagnosis between SBCC and non-aggressive BCC. Based on our analysis, we suggest that the diagnostic accuracy and primary clinic conjecture of SBCC is quite low compared to other forms of BCC such as nodular, superficial and pigmented. The combination of dermoscopy-reflectance confocal microscopy image evaluation has been found to significantly improve the accuracy and safety threshold in equivocal “pink” cutaneous lesions in the differential diagnosis of BCC.23 So that, considering that SBCC is a distinct entity from other subtypes of BCC and its delayed diagnosis has a negative clinical outcome, we suggest that more accurate diagnostic tools apart from dermatoscopy are required to reach the goal of an early-stage diagnosis of SBCC.
The main limitation of this study is the retrospective design. Thus, larger prospective researches are necessary to confirm our findings.
Examining factors associated with delayed diagnosis is complex because many are inter-related21. However, the single-center research design of our study gives the chance of making interesting inferences since all cases were diagnosed using the same diagnostic tool for both subgroups of SBCC and non-aggressive BCC. The confounding effect of age was doubly controlled: a) by stratifying by decades and b) by adjusting for older vs younger patients. Our sample has a remarkable size and provides a good representation of the reference population. Besides, the power of our study is considerably high to give strength to our observations. Nevertheless, caution should be taken in generalizing these outcomes, because they are based on results from a single centre.
ConclusionAs a distinct entity, SBCC is diagnosed with dermatoscopy later when compared to less aggressive subtypes of BCC. Gender is not important in explaining the difference observed.
Financial support: None.
Conflict of interest: None.