Several dermatoses are mediated by histamine, such as urticaria, angioedema, and papular urticaria. There are no Brazilian studies comparing the potency of antihistamines.
Objectives:To evaluate the tolerability and efficacy of the main commercial brand and generic H1 antihistamines, regarding the suppression of the wheal and flare to the histamine test.
Methods:A quasi-experimental, open study with 10 healthy adults submitted to the histamine test on the ventral aspect of the forearms. After 20 minutes, wheal and flares were measured. The tests were performed after two hours of intake of dexchlorpheniramine, hydroxyzine, levocetirizine, fexofenadine, cetirizine, loratadine, ebastine, desloratadine, epinastine and rupatadine, as well as generics of loratadine, cetirizine and fexofenadine.
Results:All antihistamines presented a reduction in the wheal compared to the control (p <0.02), as well as in the flare, except for rupatadine (p = 0.70). In the internal comparison, cetirizine, fexofenadine, epinastine, levocetirizine, dexchlorpheniramine and hydroxyzine were the most potent, with no difference between them (p > 0.1). As for halo, cetirizine, epinastine, hydroxyzine and fexofenadine were the most potent, with no difference between them (p > 0.1). The most common adverse effect was drowsiness, which was more prevalent among first-generation drugs (p < 0.01). Generic loratadine, fexofenadine and cetirizine halos were higher than their controls (p >0.03)..
Study limitations:A single-center study evaluating only aspects related to histamine.
Conclusions:Brazilian commercial antihistamines presented different profiles of inhibition of wheal and flares in the histamine test, as well as adverse effects. Generic loratadine, fexofenadine and cetirizine presented larger flares than brand drugs.
Many skin conditions are mediated by histamine, such as urticaria (physical and immunomediated) angioedema and papular urticaria, supporting the frequent use of type I histamine receptor blockers (AH) in dermatology.1,2
Urticaria is the main histamine-mediated condition in dermatology; it is common and affects patient’s quality of life.3 Its prevalence is estimated in 1-1.5%, and up to 10-15% of the population will have one episode sometime in their life.4,5
The main effector cell in the physiopathology of most causes of urticaria is the mast cell, that releases mediators such as histamine, inflammatory cytokines, chemokines, leukotrienes, prostaglandins and platelet-activating factor upon degranulation. These mediators are responsible for vasodilation, sensory activation, plasma leakage and recruitment of cells for the site of these lesions.6
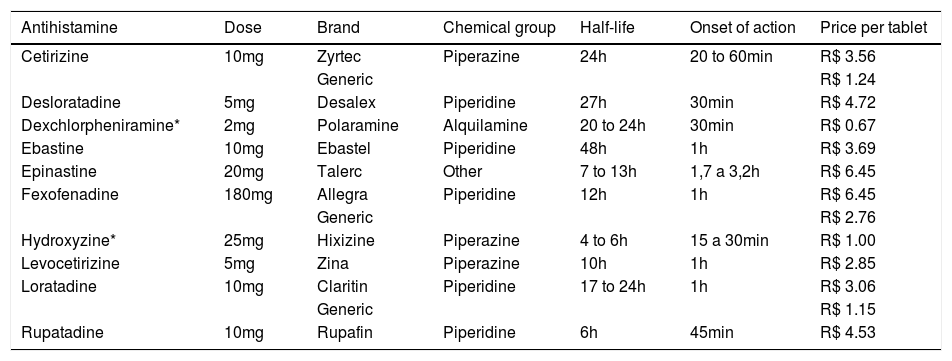
AH show good absorption when administered orally and, therefore, are capable of reaching effective plasma levels in less than two hours after intake (Table 1). Most are metabolized in the liver and excreted by the kidneys. They are divided into first and second (or more) generation AH.
Main commercially antihistamines (anti-H1) available in Brazil, their chemical groups and pharmacologic characteristics, cost and doses tested by posological unit
| Antihistamine | Dose | Brand | Chemical group | Half-life | Onset of action | Price per tablet |
|---|---|---|---|---|---|---|
| Cetirizine | 10mg | Zyrtec | Piperazine | 24h | 20 to 60min | R$ 3.56 |
| Generic | R$ 1.24 | |||||
| Desloratadine | 5mg | Desalex | Piperidine | 27h | 30min | R$ 4.72 |
| Dexchlorpheniramine* | 2mg | Polaramine | Alquilamine | 20 to 24h | 30min | R$ 0.67 |
| Ebastine | 10mg | Ebastel | Piperidine | 48h | 1h | R$ 3.69 |
| Epinastine | 20mg | Talerc | Other | 7 to 13h | 1,7 a 3,2h | R$ 6.45 |
| Fexofenadine | 180mg | Allegra | Piperidine | 12h | 1h | R$ 6.45 |
| Generic | R$ 2.76 | |||||
| Hydroxyzine* | 25mg | Hixizine | Piperazine | 4 to 6h | 15 a 30min | R$ 1.00 |
| Levocetirizine | 5mg | Zina | Piperazine | 10h | 1h | R$ 2.85 |
| Loratadine | 10mg | Claritin | Piperidine | 17 to 24h | 1h | R$ 3.06 |
| Generic | R$ 1.15 | |||||
| Rupatadine | 10mg | Rupafin | Piperidine | 6h | 45min | R$ 4.53 |
First generation AH cross the blain-blood barrier and act on muscarinic, serotonin, adrenergic receptors, among others, causing adverse effects like drowsiness, hyperactivity, insomnia and seizures.1 On the other hand, second and third generation AH, besides more potent and longer lasting, have few adverse effects because the brain-blood barrier is less permeable to them, and they have a high affinity to H1 receptors.7 There are no studies in Brazil comparing the efficacy to histamine challenge and tolerability of commercial AH.
The epicutaneous histamine test allows for a comparison of the efficacy between drugs regarding the blockage to vascular permeability (wheal) and neuro-mediated reflex vasodilation (flare) by the activation of histamine receptors in the skin.8
This study aims at evaluating the efficacy of wheal and flare suppression to the histamine test, besides the tolerability profile of the main antihistamines (anti-H1) commercialized in Brazil, and compare the performance with generic drugs.
MethodsQuasi-experimental, open, self-controlled study, approved by the Ethics Committee of the Institution (CAAEE: 58849716.4.0000.5411). Ten healthy volunteers of both genders, older than 18 years of age and younger than 60, with no past history of anaphylaxis, asthma or urticaria, non-pregnant and not breastfeeding, with no recent history of AH and corticosteroid use were included in the study.
The study was conducted at the Dermatology Outpatient Clinic of FMB-Unesp (Botucatu-SP) from June to November, 2016.
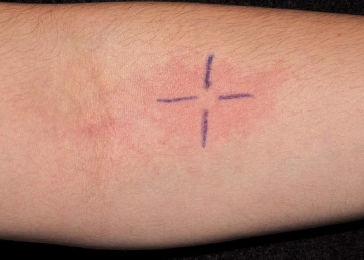
Firstly, we performed a control test. A drop of histamine (histamine dihydrochloride 1:1,000) was introduced in each forearm (2cm from the antecubital fossa) with a disposable lancet. After 20 minutes, the diameter of the papules and wheals formed was measured (Figure 1).9-11
In the subsequent tests, the same procedure was performed two hours after the intake of one of the commercial AH of the following brands: dexchlorpheniramine 6mg (Polaramine), hydroxyzine 25mg (Hixizine), levocetirizine 5mg (Zina), fexofenadine 180mg (Allegra), cetirizine 10mg (Zyrtec), loratadine 10mg (Claritin), ebastine 10mg (Ebastel), desloratadine 5mg (Desalex), epinastine 20mg (Talerc) and rupatadine 10mg (Rupafin), besides generic fexofenadine 180mg (Ranbaxy), loratadine 10mg (Merck) and cetirizine 10mg (Medley) (Table 1).
Volunteers were also questioned about possible side effects related to the medication, in particular drowsiness and dry mouth.
All tests were performed in duplicates (bilateral), in the afternoon (14h-16h), with a minimal interval of 48h so that one would not affect the other. As reference we adopted the product of the diameters of flares and wheals. The results were compared among the evaluators with a generalized linear mixed model (gamma with log link). Adherence to the probability distribution was tested by the Q-Q plot and the adjustment of the model compared by the corrected Akaike information criterium.12,13
The primary outcome was the analysis of the efficacy of antihistamines compared to control. For this test we used the Holm-Bonferroni post-hoc correction and considered significant one-sided p-values of ≤ 0,05.13
The secondary outcome was the internal comparison of efficacy between the antihistamine groups among themselves and brand medications with their generic. For these tests we used the Holm-Bonferroni post-hoc correction and considered significant two-sided p-values of ≤ 0,05.13
The comparison between the frequencies of adverse effects between AH groups was tested using McNemar, chi-square and Fisher’s exact tests.13
The concordance between the values of the forearms was calculated by the Intraclass Correlation Coefficient (ICC) for a perfect concordance.14 The correlation between the values of flares and wheals was assessed by Spearman’s correlation coefficient (rho).13
Sample size was calculated after a pre-test with 10 volunteers in order to detect a difference of up to 5mm in the wheal of the histamine test in comparison to control.15
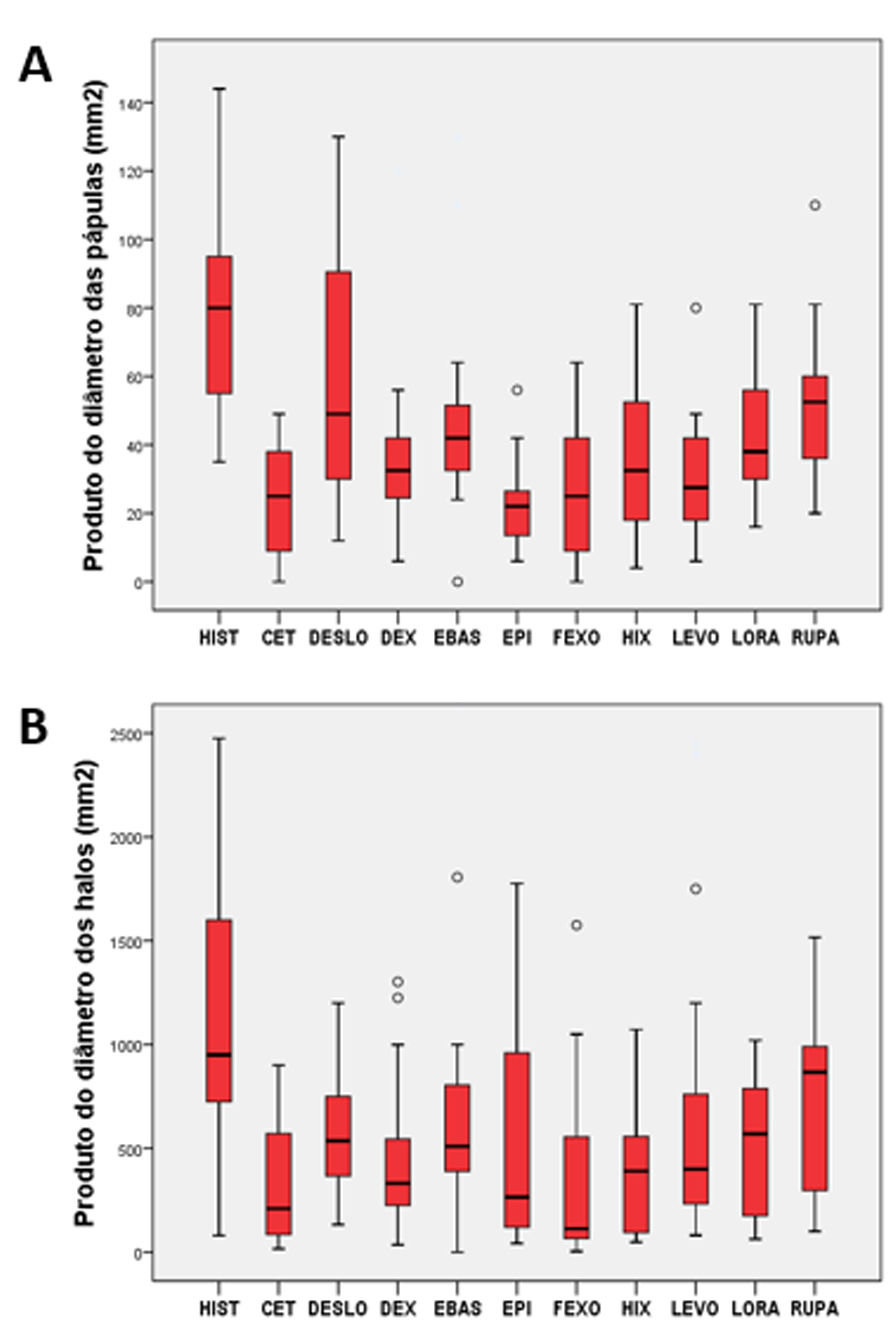
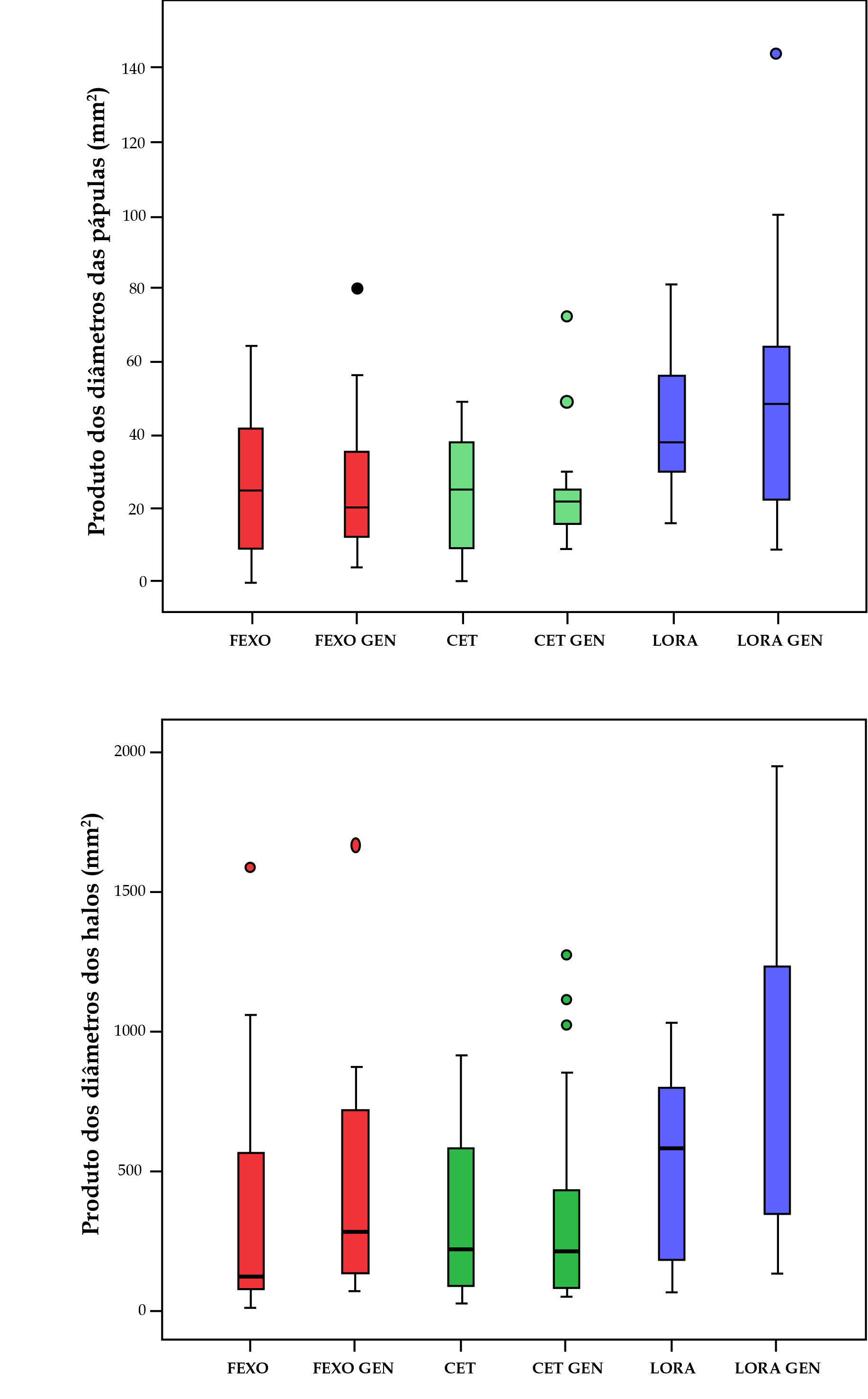
ResultsThe cases were made up of seven female subjects and three male subjects, with ages between 23 and 51 years. Figures 2 and 3 show the values of the measurement of wheals and flares of the histamine tests with brand and generic AH.
There was a high concordance between the values of flares (ICC = 0.89; p < 0.01) and a substantial concordance between wheals (ICC = 0.67; p < 0.01) for the right and left forearm, however, there was no correlation between the diameter of flares and wheals for the histamine test (rho = 0.20; p = 0.39).
All AH showed a profile of wheal reduction in comparison to control (p < 0.02), as well as flares, except for rupatadine (p = 0.70) (Figure 2).
In the internal comparison, regarding wheal suppression, cetirizine, fexofenadine, epinastine, levocetirizine, dexchlorpheniramine and hydroxyzine were the most potent, with no difference between them (p > 0.1); the worst performances when compared to the other AH tested, were related to desloratadine, ebastine and rupatadine (p < 0.05) (Figure 2). Loratadine showed an intermediate potency and a variable significance among AH.
Regarding suppression of flare, cetirizine, epinastine, hydroxyzine and fexofenadine were the most potent, with no difference between them (p > 0.1); the worst performances when compared to the other AH were related to desloratadine and ebastine (p < 0.05) (Figure 2). Loratadine, rupatadine and dexchlorpheniramine show intermediate potency and variable significance among AH.
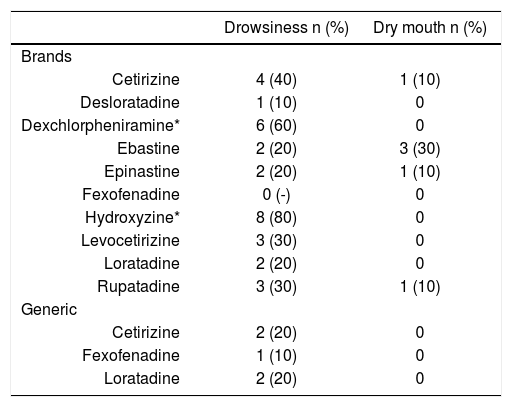
The most commonly reported adverse effect was drowsiness, more prevalent between first generation AH (70% vs 21%; p < 0.01). Dry mouth was not significantly different between the groups (0% vs 8%; p = 0.34) (Table 2).
Frequency of drowsiness and dry mouth between the antihistamine tested (n = 130)
| Drowsiness n (%) | Dry mouth n (%) | |
|---|---|---|
| Brands | ||
| Cetirizine | 4 (40) | 1 (10) |
| Desloratadine | 1 (10) | 0 |
| Dexchlorpheniramine* | 6 (60) | 0 |
| Ebastine | 2 (20) | 3 (30) |
| Epinastine | 2 (20) | 1 (10) |
| Fexofenadine | 0 (-) | 0 |
| Hydroxyzine* | 8 (80) | 0 |
| Levocetirizine | 3 (30) | 0 |
| Loratadine | 2 (20) | 0 |
| Rupatadine | 3 (30) | 1 (10) |
| Generic | ||
| Cetirizine | 2 (20) | 0 |
| Fexofenadine | 1 (10) | 0 |
| Loratadine | 2 (20) | 0 |
When brand medications were compared to their generics, there was difference between the values of flare for fexofenadine (p < 0.01), cetirizine (p < 0.01) and loratadine (p = 0.02); however, there was no difference between the wheal values (p > 0.1) (Figure 3). The frequency of drowsiness and dry mouth were not different between the groups (p > 0.50) (Table 2).
DiscussionThere was a great variability in the suppression profiles of flare and wheal to the histamine test, as well as adverse effects between AH in the dose and regime tested, and even between the volunteers, what reflects different response patterns found in clinical practice.
Other studies with slightly different methodologies confirm our results regarding the superiority of cetirizine, fexofenadine, epinastine, levocetirizine, dexchlorpheniramine and hydroxyzine in the suppression of the histamine-induced wheal.9,10,16-20 These data do not discredit the efficacy of the other AH tested, since they effectively suppressed the wheal in comparison to control, however, our results can influence in the decision to change AH in cases of unsatisfactory control of the condition.
The triple response of Lewis, described almost a century ago, assumes that the wheal and flare formation to the histamine test are independent phenomena that depend on vascular and neurologic integrity.21 Papules are mainly formed by interstitial edema and should correlate to the intensity of wheals and rhinitis effusion, being the most clinically relevant measurement.10 The flare is a vasodilation phenomenon that depends on the neural reactivity and can correlate to the pruritus. In fact, there was no correlation between the diameters of the flare and the wheal for the patients in this study, confirming their independence. As different AH show individualized performances regarding the suppression of the wheal and the flare, this study subsidizes possibilities of therapeutic success of different drugs in different histamine-mediated conditions.
The reactivity to the histamine test should not be interpreted as absolute, since the individual response can vary according to circadian rhythm, underlying infections/inflammations, room temperature, site of the test, neurologic integrity, other drugs and emotional stress levels.22,23 The study design using repeat measurements in both forearms, at the same time and in a homogenous group of healthy volunteers favors the internal validity of the results.
Our data add new information in Brazil regarding the potency of histamine blockage. The choice of AH should contemplate different clinical, pharmacologic, economic, dosing aspects and side effects. Even drowsiness, the main side effect of first generation AH can be strategic in pruritic conditions of central origin, such as uremic pruritus from hemodialysis, and its safety profile permissive in this group of patients.24
Generics were regulated in Brazil in 1999 as drugs with proven bioequivalence in laboratories certified by ANVISA. There is a concern of the medical community that the bioequivalence of the active ingredient does not ascertain adequate bioavailability, solubility and pharmacokinetics as reference brand drugs.25-29
Generic AH drugs showed some discrepancies regarding suppression potency of flares when compared to brand medications, however, there was no difference regarding wheals, what is more relevant clinically.10 These results should raise attention for the possibility that therapeutic failures could be due to intrinsic properties of a specific generic preparation.26,29,30
The study shows limitations related to the investigation of healthy individuals, single center, in a controlled situation, with only histamine challenge and a single dose of AH; this favors the internal comparison of the drugs but does not take into consideration the inflammatory and psychogenic aspects involved in histamine-dependent conditions.8,31,32
There is a large variety of generic and similar AH in the Brazilian market. The choice of these manufacturers was due to the lowest price at the moment of purchase and their results do not allow generalization for other brands, which should be subsequently investigated.
Also, some AHs are known to be anti-inflammatory and act in the synthesis of leukotrienes and prostaglandins, and can have a more favorable performance in inflammatory and pruritic conditions than in this experimental comparison.33
Clinical trials should be performed in order to counterproof these results and, in addition, future comparisons should consider the association between AH, consecutive day use, variation in doses, combination with H2 and H3 receptor blockers, besides mast cell membrane stabilizers, since they are also strategies used for the treatment of refractory urticaria.34,35
ConclusionThe main Brazilian commercial AH showed different profiles of flare and wheal suppression in the histamine test, as well as of adverse effects. Loratadine, fexofenadine and cetirizine showed different flare profiles among the brand and generic medications tested. The choice of the drug for treatment of histamine-mediated conditions should take into account clinical, tolerability and pharmacoeconomic aspects.
Financial support: None.
Conflict of interest: None.