Psoriasis has been associated with co-morbidities and elevated cardiovascular risk.
Objectives:To analyze the relationships among metabolic syndrome, cardiovascular risk, C-reactive protein, gender, and Psoriasis severity.
Methods:In this cross-sectional study, plaque Psoriasis patients (n=90), distributed equally in gender, were analyzed according to: Psoriasis Area and Severity Index, cardiovascular risk determined by the Framingham risk score and global risk assessment, C-reactive protein and metabolic syndrome criteria (NCEPT-ATP III).
Results:Metabolic syndrome frequency was 43.3% overall, without significance between genders (P=0.14); but women had higher risk for obesity (OR 2.56, 95%CI 1.02-6.41; P=0.04) and systemic arterial hypertension (OR 3.29, 95%CI 1.39-7.81; P=0.006). The increase in the Psoriasis Area and Severity Index also increased the risk for metabolic syndrome (OR 1.060, 95%CI 1.006-1.117; P=0.03). Absolute 10-year cardiovascular risk was higher in males (P=0.002), but after global risk assessment, 51.1% patients, 52.2% women, were re-classified as high-intermediate cardiovascular risk; without significance between genders (P=0.83). C-reactive protein level was elevated nearly six-fold overall, higher in metabolic syndrome (P=0.05), systemic arterial hypertension (P=0.004), and high-intermediate 10-year cardiovascular risk patients (P<0.001); positively correlated to: Framingham risk score (P<0.001; r=0.60), absolute 10-year cardiovascular risk (P<0.001; r=0.58), and age (P=0.001; r=0.35); but not to Psoriasis Area and Severity Index (P=0.14; r=0.16); increased the 10-year cardiovascular risk (R2=33.6; P<0.001), MetS risk (OR 1.17, 95%CI 0.99-1.37; P=0.05) and with age (P=0.001). HDL-cholesterol level was higher in normal C-reactive protein patients (t=1.98; P=0.05).
Study limitations:Restricted sample, hospital-based and representative of a single center and no specification of psoriatic arthritis.
Conclusions:Psoriasis, metabolic syndrome, systemic arterial hypertension and age share the increase in C-reactive protein, which could implicate in additional burden for increasing the cardiovascular risk and be an alert for effective interventions.
Psoriasis (PsO) is an immune-mediated inflammatory disease that primarily affects skin and joints, with systemic repercussions that raise the risk of developing cardio- and cerebrovascular diseases (CCVD).1-8 Among a variety of co-morbidities, obesity, diabetes mellitus (DM), and metabolic syndrome (MetS) have been consistently associated with PsO.8-14
MetS comprises a set of risk factors for CCVD diseases and DM, including central obesity, atherogenic dyslipidaemia, systemic arterial hypertension (SAH) and insulin resistance.2,8,11-14 Nevertheless, the prevalence of MetS varies by gender, age, race, ethnicity and geographic region of the study population, as a function of the chosen definition, International Diabetes Federation (IDF) or National Cholesterol Education Program - Adult Treatment Panel III (NCEP-ATP III).15-18
The mechanistic links between the inflammation of PsO, metabolic alterations and increased risk of CCVD are not fully understood2,12-14 PsO attributable risk remains difficult to assess due to confounding factors.19 However, large studies indicate that the increased cardiovascular risk (CVR) is at least partially attributable to PsO and independent of the presence of metabolic co-morbidities. Severe psoriasis has been considered as an independent risk factor for acute heart attack, stroke and cardiovascular mortality when adjusting for major risk factors.4,5,20,21
C-reactive protein (CRP) is an acute phase protein that can be elevated in both infectious and inflammatory processes.22 There are strong evidences of relationship between CRP and coronary heart disease (CHD), and also records of elevated CRP levels on MetS.23,24
Elevated CRP levels and other inflammatory markers have been associated with skin disease activity of PsO, mainly psoriatic arthritis (PsA) and generalized pustular PsO.25-29
This study had the purpose to evaluate the prevalence of MetS and its components, according to gender and PsO severity, and the relationships among MetS, CRP and CVR in PsO patients.
MethodsSubjectsWe conducted a cross-sectional hospital-based study after its approval by the Ethics Committee of the Hospital of Clinics of School of Medicine of Ribeirão Preto, University of São Paulo, Brazil, for which were sequentially selected 90 adult subjects with plaque PsO in follow-up at Dermatology outpatient clinic. The severity of PsO was determined using the Psoriasis Area and Severity Index (PASI), according to score values 0 to 72: mild PsO was defined as PASI ≤ 10 and moderate to severe PsO defined as PASI > 10.30,31
Collection of data, blood samples and laboratory assaysThe collection of anthropometric data (weight, height, waist circumference) and measurement of systemic blood pressure were obtained by physical examination according to standard procedures. The waist circumference (cm) was performed with flexible tape by positioning at half of the distance between the iliac crest and lower costal border. Systemic blood pressure (BP), diastolic and systolic (mmHg), was measured by analogical sphygmomanometer (Tycos, Welch Allyn, Skaneateles Falls, NY, USA) in the right arm, in the sitting position and after five minutes of rest, and at least two measures were performed for the calculation of mean. The collected blood samples were obtained after fasting of at least 12 hours. The dosage of fasting glucose (mg/dL) was performed using the glucose-oxidase method; cholesterol, high-density lipoprotein (HDL)-cholesterol and triglyceride (mg/dL) by enzyme-dosing colorimetric method and CRP (mg/dL) by immunoturbidimetry method, with reference value less than 0.5 mg/dL.
Criteria of metabolic syndrome and cardiovascular riskCriteria used for the diagnosis of MetS were those recommended by the NCEP-ATP III, which is defined by the presence of at least three of these components: 1) increased waist circumference (> 102 cm for men, > 88 cm for women); 2) elevated triglycerides (≥ 150 mg/dL) or use of triglyceride-lowering drugs; 3) low HDL- cholesterol (< 40 mg/dL in men, < 50 mg/dL in women); 4) SAH (≥ 130/ ≥ 85 mmHg) or use of antihypertensive drugs; and 5) fasting glucose (≥ 110 mg/dL) or use of antidiabetic drugs.18
Absolute CVR within ten years of the subjects was determined by the Framingham risk score (FRS), values under 10% were classified as low risk; between 10% to 20%, intermediate risk, and above 20% as high-risk for fatal or non-fatal myocardial infarction events. In the global risk assessment, according to NCEPT- ATP III, all patients with DM or existing coronary heart disease (CHD) were categorized as high risk (CHD high-risk equivalents), as well as other manifestations of atherosclerosis, event or procedure previously performed, according to patient file or patient report. MetS and family history of premature CHD (CHD in male first-degree relative < 55 years of age; CHD in female first-degree relative < 65 years of age) were considered risk-enhancer factors.19 Low and intermediate-risk patients with risk enhancers were re-classified one category above of CVR estimated singly by FRS.
Statistical analysisMetS, global CVR (high/intermediate/low), gender and CRP (normal/elevated) as dependent variables had their relationships with independent variables analyzed by Chi-squared test. Comparisons of CRP levels (mg/dL), FRS and absolute 10-year CVR (%) between genders and two PASI categories (≤10/ >10) were assessed by nonparametric Mann-Whitney test, because these variables rejected the normality hypothesis of Kolmogorov-Smirnov; CRP levels (mg/dL) in age (≤ 40 years / >40 years), MetS (no/yes) and SAH (no/yes) categories by t-test; global CVR (high/intermediate/low) and CRP levels (mg/dL) interactions by ANOVA and Bonferroni post-hoc test; relationships between CRP levels (mg/dL) and each numerical variable: age (years), PASI scores, FRS, absolute 10-year CVR (%) by Pearson’s R correlation test; and relationships between one categorical or numerical dependent variable and independent variables were assessed by multivariate logistic or linear regression analysis, respectively. The significance level α ≤ 0.05 was considered, and odds ratio (OR) was established with confidence interval (CI) of 95%. The data were analyzed through SPSS Statistics for Windows, Version 17.0 Chicago: SPSS Inc.
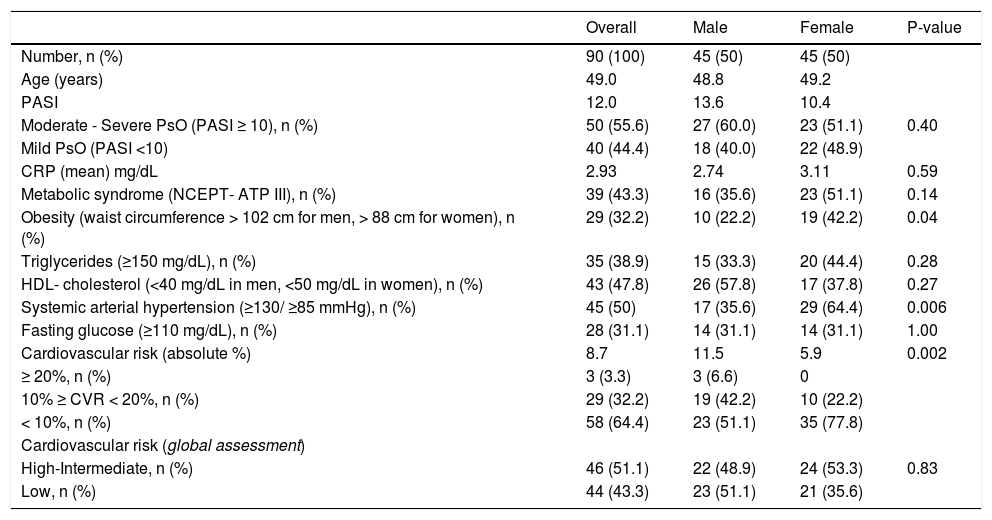
ResultsComorbidity frequencies and risksNinety PsO patients equally distributed between males and females, with the mean age of 49.0 years-old ± 12.84; 48.8 for men and 49.2 for women, respectively, were analyzed. The mean duration of illness was 12.2 years and 55.6% had severe to moderate PsO (Table 1 and 2). The prevalence of MetS in the overall sample was 43.3%, without statistical difference in the comparison between genders (female 51.1% vs. male 35.6%; P = 0.14; OR 1.89, 95% CI 0.81 - 4.35). However, women had a strong tendency for MetS risk (OR 2.35, 95% CI 0.96 -5.75; P = 0.06) in the multivariate logistic regression analysis with gender and age (years), PASI scores and CRP (mg/dL) values as predictor variables. In addition, women had nearly 2.5-fold risk for obesity (OR 2.56, 95% CI 1.02 - 6.41) and threefold risk for SAH (OR 3.29, 95% CI 1.39 - 7.81): obesity (42.2% vs 22.2%; P = 0.04) and SAH frequencies (64.4% vs 35.6%; P = 0.006) were significantly higher in women; but there were no significant differences of DM and severe-moderate PsO frequencies; and triglycerides and HDL-cholesterol levels between males and females (Table 1).
Metabolic syndrome, cardiovascular risk and PASI-defined disease severity parameters according to genders of psoriasis patients
| Overall | Male | Female | P-value | |
|---|---|---|---|---|
| Number, n (%) | 90 (100) | 45 (50) | 45 (50) | |
| Age (years) | 49.0 | 48.8 | 49.2 | |
| PASI | 12.0 | 13.6 | 10.4 | |
| Moderate - Severe PsO (PASI ≥ 10), n (%) | 50 (55.6) | 27 (60.0) | 23 (51.1) | 0.40 |
| Mild PsO (PASI <10) | 40 (44.4) | 18 (40.0) | 22 (48.9) | |
| CRP (mean) mg/dL | 2.93 | 2.74 | 3.11 | 0.59 |
| Metabolic syndrome (NCEPT- ATP III), n (%) | 39 (43.3) | 16 (35.6) | 23 (51.1) | 0.14 |
| Obesity (waist circumference > 102 cm for men, > 88 cm for women), n (%) | 29 (32.2) | 10 (22.2) | 19 (42.2) | 0.04 |
| Triglycerides (≥150 mg/dL), n (%) | 35 (38.9) | 15 (33.3) | 20 (44.4) | 0.28 |
| HDL- cholesterol (<40 mg/dL in men, <50 mg/dL in women), n (%) | 43 (47.8) | 26 (57.8) | 17 (37.8) | 0.27 |
| Systemic arterial hypertension (≥130/ ≥85 mmHg), n (%) | 45 (50) | 17 (35.6) | 29 (64.4) | 0.006 |
| Fasting glucose (≥110 mg/dL), n (%) | 28 (31.1) | 14 (31.1) | 14 (31.1) | 1.00 |
| Cardiovascular risk (absolute %) | 8.7 | 11.5 | 5.9 | 0.002 |
| ≥ 20%, n (%) | 3 (3.3) | 3 (6.6) | 0 | |
| 10% ≥ CVR < 20%, n (%) | 29 (32.2) | 19 (42.2) | 10 (22.2) | |
| < 10%, n (%) | 58 (64.4) | 23 (51.1) | 35 (77.8) | |
| Cardiovascular risk (global assessment) | ||||
| High-Intermediate, n (%) | 46 (51.1) | 22 (48.9) | 24 (53.3) | 0.83 |
| Low, n (%) | 44 (43.3) | 23 (51.1) | 21 (35.6) |
PsO, Psoriasis; PASI, Psoriasis Area and Severity Index; CRP, C-reactive protein; NCEP-ATP III, National Cholesterol Education Program - Adult Treatment Panel III; HDL, high-density lipoprotein.
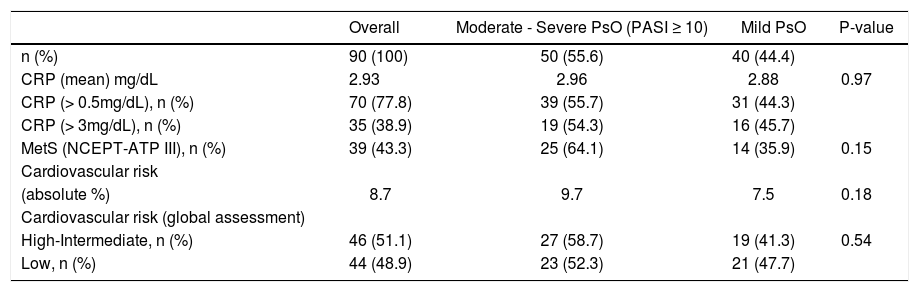
Relationships between PASI-defined disease severity, C-reactive protein, metabolic syndrome and cardiovascular risk in psoriasis patients
| Overall | Moderate - Severe PsO (PASI ≥ 10) | Mild PsO | P-value | |
|---|---|---|---|---|
| n (%) | 90 (100) | 50 (55.6) | 40 (44.4) | |
| CRP (mean) mg/dL | 2.93 | 2.96 | 2.88 | 0.97 |
| CRP (> 0.5mg/dL), n (%) | 70 (77.8) | 39 (55.7) | 31 (44.3) | |
| CRP (> 3mg/dL), n (%) | 35 (38.9) | 19 (54.3) | 16 (45.7) | |
| MetS (NCEPT-ATP III), n (%) | 39 (43.3) | 25 (64.1) | 14 (35.9) | 0.15 |
| Cardiovascular risk | ||||
| (absolute %) | 8.7 | 9.7 | 7.5 | 0.18 |
| Cardiovascular risk (global assessment) | ||||
| High-Intermediate, n (%) | 46 (51.1) | 27 (58.7) | 19 (41.3) | 0.54 |
| Low, n (%) | 44 (48.9) | 23 (52.3) | 21 (47.7) |
PsO, Psoriasis; PASI, Psoriasis Area and Severity Index; MetS, Metabolic syndrome; CRP, C-reactive protein; NCEP-ATP III, National Cholesterol Education Program - Adult Treatment Panel III.
Severe-moderate and mild PsO groups showed MetS frequencies without statistical significance (64.1% vs. 35.9%; P = 0.15) (Table 2). There was a tendency of a higher MetS risk in patients older than 40 years compared to those aged 40 years or younger (OR 2.51, 95% CI 0.88 -7.20; P < 0.08), but without statistical significance. In the multivariate logistic regression analysis with gender, age (years), CRP (mg/dL) and PASI scores as predictor variables, each one PASI unit increased in 6% average the risk for MetS (OR 1.060, 95% CI 1.006 - 1.117; P = 0.03). The CRP mean level was significantly higher in patients with MetS (t = 1.98; P = 0.05); and SAH (t = 2.96; P=0.004) when all MetS criteria were analyzed. The linear regression analysis of the relationships among the CRP levels (mg/dL), age (years), PASI scores, gender and MetS showed the CRP value increased in 1.02 average with MetS presence (P = 0.05); and 0.07 average each year of age (P = 0.001).
CRP levels, PASI scores, age and HDL- cholesterol abnormalitiesIn the sample, CRP level was elevated in 77.8% of patients, and the 2.93mg/dL mean value of CRP was 5.86-fold higher than reference value (0.5mg/dL). Regarding the CRP levels, there were no statistical differences in comparison between genders (female 3.11mg/dL x male 2.74mg/dL, respectively, Z = 0.54; P = 0.59) (Table 1), and moderate to severe and mild PsO patients (2.96 mg/dL vs. 2.88mg/dL, respectively; Z = 0.03; P = 0.97) (Table 2). Thirty-five patients had CRP > 3mg/dL, among them 45.7% presented mild PsO and 10 men (28.57%) had CVR more than or equal to 20% (Table 2). The CRP levels were positively correlated to age (r = 0.35; P = 0.001), but not to the PASI scores (r = 0.16; P = 0.14). Patients older than 40 years compared to 40-year-old or younger showed higher frequency (82.9% x 50.0%; P = 0.003, X2 = 9.09), and nearly fivefold more chances to have elevated levels of CRP (OR 4.83, 95% CI 1.65 - 14.16; P = 0.004) in the logistic regression analysis that included the categorical variables age (≤ 40 years / >40 years), gender, PASI score (≤ 10 or >10) and MetS. The HDL-cholesterol mean level was significantly higher in patients with CRP normal levels (t = 1.98; P = 0.05); and in the logistic regression analysis, the increase of each HDL-cholesterol one unit reduced, in 5% average the risk of CRP abnormality (OR 0.95, 95% CI 0.90 - 1.00; P = 0.056). The relationship analyses among the CRP, TG and HDL-cholesterol levels, abdominal circumference and PASI values, age, gender and SAH showed CRP values increased in 1.26 average with SAH presence (P = 0.015) and in 0.067 average each year of the age (P = 0.001); and reduced in 0.049 average each increase of HDL-cholesterol one unit (P = 0.062).
Cardiovascular risk assessmentAbsolute 10-year CVR average was 8.7% in overall and significantly higher in male compared to female (11.5% vs. 5.9%; Z = 3.12; P = 0.002). However, after global risk assessment, which considered high-risk equivalents and risk-enhancer factors, 46 (51.1%) patients (22 males and 24 females) were re-classified as high-intermediate CVR; then no statistical significance of the 10-year CVR (P = 0.83) was observed in the gender comparison (Table 1). In severe to moderate and mild PsO patients, the absolute 10-year CVR was 9.7% and 7.5%, respectively, with no statistical significance (P = 0.18) (Table 2). In the multivariate logistic regression analysis with categorized age (≤ 40 years/ > 40 years), only the variables obesity (P = 0.002; OR 13.18, 95% CI 2.63 - 66.10); triglyceride level (P = 0.007; OR 5.44, 95% CI 1.58 - 18.82) and SAH (P = 0.02; OR 3.98, 95% CI 1.29 - 12.21) exerted simultaneous and independent effects on CVR.
CRP levels, metabolic syndrome and cardiovascular riskCRP mean values were significantly higher: i) in patients with MetS compared to those without MetS (3.55 mg/dL vs. 2.44 mg/dL; P = 0.05); and ii) in both patients with high and intermediate compared to low 10-year CVR (P < 0.001) but, were similar in high and intermediate 10-year CVR groups (P = 0.55). One-unit increase of CRP increased in 17% the chances of an individual with PsO to have MetS (OR 1.17 95% CI 0.99 - 1.37; P = 0.05), and 1.68% average of the absolute 10-year CVR (R2 = 33.6; P < 0.001). Furthermore, there was a positive correlation between CRP level and FRS (P < 0.001; r = 0.60), and absolute 10-year CVR values (P < 0.001; r =0.58).
In a subsample (n=72), the CRP average was slightly higher among individuals under topical treatments compared to those using systemic therapies, but with no statistical significance (3.15 mg/dL vs 2.70 mg/dL; P > 0.05); also, absolute 10-year CVR (9.2% vs 9.5%) and MetS frequencies (31.0% vs. 25.6%; P=0.61), respectively, were similar in these groups.
DiscussionOur study emphasizes the relevance of the identification and monitoring of risks for CCVD in PsO patients, and the need of biomarkers related to activity disease, risks and comorbidities.
The increased proliferation of keratinocytes is caused by an immunopathological process which involves T cells, antigen-presenting dendritic cells, Langerhans cells, neutrophils and pro-inflammatory cytokines (TNF-alpha, IL-12/-23, IL-17 and IFN-gama).1 Additionally, a low-grade systemic inflammatory state has been associated with several comorbidities significantly prevalent in PsO patients. Obesity has been related to increased production of pro-inflammatory cytokines (TNF-alpha, IFN-gama, IL-2, IL-1, IL-6 and IL-8), and of CRP, which are accountable for some aspects of the insulin resistance, hypercoagulability and endothelial dysfunction associated with atherosclerosis.14,32-34
Raised levels of CRP, IL-6 and TNF-alpha have been related to conditions such as smoking, depressive symptoms, and psychosocial stress, which unidirectional and bidirectional relationships with inflammation have been established. 35-37 Additionally, the elevation of these inflammatory markers has also been associated with some age-related chronic diseases making these relationships non-specific.38
CRP plays a pro-atherogenic and pro-thrombotic role. Elevated CRP concentration or CRP mRNA can be observed in atherosclerotic plaques, adipose tissue, and CRP synthesis seems to be positively related to leptin levels.39
Raised high sensitivity CRP levels have been consistently associated with CHD and elevated CVR, and related to cerebrovascular disease, MetS and PsO.23-29,40
Ultimately, as in an interlaced circuit, sustained levels of PsO inflammatory mediators would play a role in the precipitation, maintenance and/or reciprocal relationship of injury with the comorbidities, particularly obesity, insulin resistance, MetS and CCVD.14,32,40
Firstly, these comorbidities need to be recognized beyond the skin involvement, because they potentially represent an extra burden for PsO patients and several are modifiable by interventions.
MetS prevalence has been distinguished across gender, race and ethnic subgroups.15-17 In the United States, by the NCEP definition, the estimated MetS prevalence ranges between 24 to 28% of adult men and 23% to 34% of adult women; it was highest for Mexican Americans and lowest for Blacks of both sexes.15,16
In a systematic review that included ten cross-sectional studies in Brazilian population, the mean for MetS prevalence Brazil was 29.6% (range 14.9%-65.3%).41 Among them, two cross-sectional population-based Brazilian studies revealed MetS prevalence of 29.8% and 35.9%,42,43 by NCEP-ATP III criteria, respectively. MetS was increased from the youngest (26-34 y) to the oldest (55-64 y) group (15.8% and 48.3%, respectively), as well as in women from the higher to the lowest socioeconomic level.42 MetS seems highly prevalent in Brazil and significantly associated with age, body mass index, and educational level.41-43
A previous hospital-based case-control study demonstrated significantly higher frequency of MetS -NCEP-ATP III criteria- in Italian patients with PsO and 40 years or older compared to the controls with other skin disorders (30.1% vs. 20.6%).44
In a previous study in Brazilian PsO patients, MetS, SAH and DM frequencies were 44.9%, 43.7% and 15.3 %, respectively.45 In our sample, MetS frequency was almost similar (43.3%), but SAH (50%) and DM (31.1%) frequencies were higher. Therefore, the two latter components were twofold and fivefold higher compared to Brazilian population, estimated in 21.4% and 6.2%, respectively.46
In our study, the MetS frequency of 47.8% in patients older than 40 years seems higher. This age group and women showed a strong tendency for MetS. In fact, women showed higher risk for SAH and obesity, and the risk for Mets was related to an increase of the levels of PsO severity and CRP. Besides PsO severity, also MetS, SAH and age showed a close relationship with an increase in CRP value.
However, comparable MetS frequencies and CRP levels found in moderate to severe or mild PsO forms suggested the potential of long life-time and systemic effects of disease, despite of its severity. Persistently elevated CRP levels in mild PsO patients, mainly those under topical treatment, is an alert to investigate other morbidities, PsA, Mets and its components, and/or start effective systemic therapies for PsO and/or PsA; and interventions indicated for other conditions (SAH, obesity, dyslipidemia, smoking, psychosocial stress, depression).
Elevated levels of CRP have been described in individuals with MetS not affected by PsO and were probably related to the inflammatory nature of the MetS itself.24 CRP levels could raise dramatically in the coexistence of PsO and MetS, and potentially increase the CVR, even to be an additional criterion for follow-up and early interventions.
High-sensitive CRP has been indicated as an additional criterion to the classical risk factors, to further assess risk for CHD and plan its primary prevention.23,47
Since risk equivalents (DM, atherosclerotic disease) and risk enhancers (MetS) are significantly prevalent in PsO patients, they must be considered in these patients, as the CVR global assessment, which adds these parameters to CVR established by FRS. Following CVR global assessment, the CVR changed to a predominance of high-intermediate CVR patients (51.1%), due particularly to the increased frequency of women (52.2%) that exceeded men frequency (47.8%) with high-intermediate CVR.
Ten-year CVR was similar in moderate to severe or mild PsO in our sample. Positive correlation between CRP level and FRS and CVR reinforce the role of CRP as predictor factor for CVR in PsO.
Elevated CRP levels support the concept of systemic inflammation in PsO and its potential and multiple repercussions. CRP is a sensitive inflammation biomarker, and its stable levels and short half-life (6-8 h) make it an appropriate tool for the follow-up of inflammatory diseases.29
Due to some discrepancies among the reports, monitoring CRP plasma levels is not yet considered as a regular practice in the management of psoriatic patients, and large-scale analyses are needed, mainly those from controlled trials.29
Our study shows that moderate to severe or mild plaque PsO has a feature of systemic inflammation represented by elevated CRP levels, but there is some disagreement about its correlation with the PsO severity as measured by PASI score and value to monitor therapy.25-28
Sergeant et al. did not find difference in hs-CRP between plaque PsO and erythroderma patients, and it was raised in those with PsA. The authors did not consider hs-CRP as a suitable biomarker for the assessment of disease activity, but high CRP values in patients with plaque PsO should alert the clinician to the possibility of joint disease.27
Overall, the literature supports the role of CRP as a laboratory indicator of PsO severity and its relationship to PASI, particularly when the patient is not under the effect of any systemic treatments for at least one month and has no signs of joint involvement.29
Therefore, PsA does not necessarily correspond to the extension and severity of cutaneous involvement and may carry an extra load of inflammation.29
Even if remission is achieved by effective PsO treatment, after a marked decline, CRP values can fail to turn to the normal range.29
The remaining inflammation may play a role in disease relapses and an inflammatory biomarker could work as a possible predictor for the length of remission and chance of re-exacerbation in a therapy course.29,48
CRP cardio-toxicity and its close relationship to all-cause mortality rate(29) emphasize an approach for the most effective therapies for reducing of PsO cutaneous and joint inflammation and consequently of the CRP levels.
Hs-CRP values can define different levels of CV risk: CRP levels of <1, 1 to 3, and >3 mg/L corresponding to low-, moderate- and high-risk groups, respectively, for CV events. For risk level classification, at least two hs-CRP measurements should be performed in a metabolically stable person without obvious inflammatory or infectious conditions.47
Hs-CRP seems to be a marker of large reduction of CVR after drug interventions, but it has not been a good predictor of the extent of atherosclerotic disease. Hs-CRP routine testing may have a potential use to augment risk assessment in the identification of persons who should be considered for cardioprotective drug therapies, as well as for increased emphasis on healthy lifestyle changes.47
Furthermore, our findings of the relationship between age, HDL-cholesterol and CRP are corroborated by the literature. High levels of CRP are associated with a decrease of HDL-cholesterol and its protective effect.49,50 Also, inflammatory markers (CRP, IL-6 and TNF-alpha) have been shown to increase with age, potentially because of declining levels of sex hormones and increase in visceral adipose tissue. 38
The hospital-based and small sample provided by a single referral center, as well as the non-attendance of healthy individuals as control group for comparisons with PsO patients consist in limitations of our study. Additionally, there was no discrimination of subsamples of PsA patients, and the choice method to CRP measurement compared to hs-CRP may result in underestimate analyses of this parameter. However, it attends the study purposes in the fundamental requirements.
ConclusionsOur study indicated high prevalence of MetS, SAH, obesity and high-intermediate CVR in PsO patients, particularly in women. We underlined that equivalent and enhancing factors must be considered for CVR global assessment, which would increase the CVR estimative of these patients. CRP is an inflammatory biomarker, also its elevation can establish a close and imbricated relationship between PsO, MetS, SAH, age, HDL-cholesterol and CVR. It is accessible to be adopted in routine and a warning for additional care in the primary prevention and/or decision-making for effective therapy use in cardiovascular disease.
According to our results, the high levels of CRP are shared by PsO and other comorbidities, whose coexistence can establish common consequences and implicate in additional burden for CVR raise.
AcknowledgmentsThe research leading to these results has received support from the Center for Research in Inflammatory Diseases [CRID] under grant agreement n°11.1.21625.01.0.
Financial support: This research has received support from the Center for Research in Inflammatory Diseases [CRID] under grant agreement n°11.1.21625.01.0.
Conflict of interest: None.