Subcorneal pustular dermatosis is a rare pustular eruption which occurs mainly in middle-aged women and rarely during childhood. We report a case of a 15-year-old female with a 4-year history of pustular lesions on the proximal region of the upper limbs with subsequent impairment of the trunk. Physical examination revealed small pustules distributed on the trunk and proximal region of the limbs. Histopathology showed a subcorneal pustule and direct immunofluorescence for IgA, IgM, IgG and fibrinogen was negative, confirming the diagnosis of subcorneal pustular dermatosis. The patient was treated with dapsone with good clinical response after one month. Subcorneal pustular dermatosis is a rare condition and there are only isolated cases reported in the literature in pediatric patients. Thus, we discuss the main clinical aspects and treatment response of this condition during childhood.
Subcorneal pustular dermatosis (SPD), also known as Sneddon-Wilkinson´s disease, is a rare, chronic, relapsing pustular eruption.1 It is clinically relevant given its association with malignant neoplasms such as multiple myeloma, chronic lymphocytic leukemia and thymoma.2
This condition is more common in women over 40 years of age and few cases have been reported in children.3 We report a case of a teenager diagnosed with SPD.
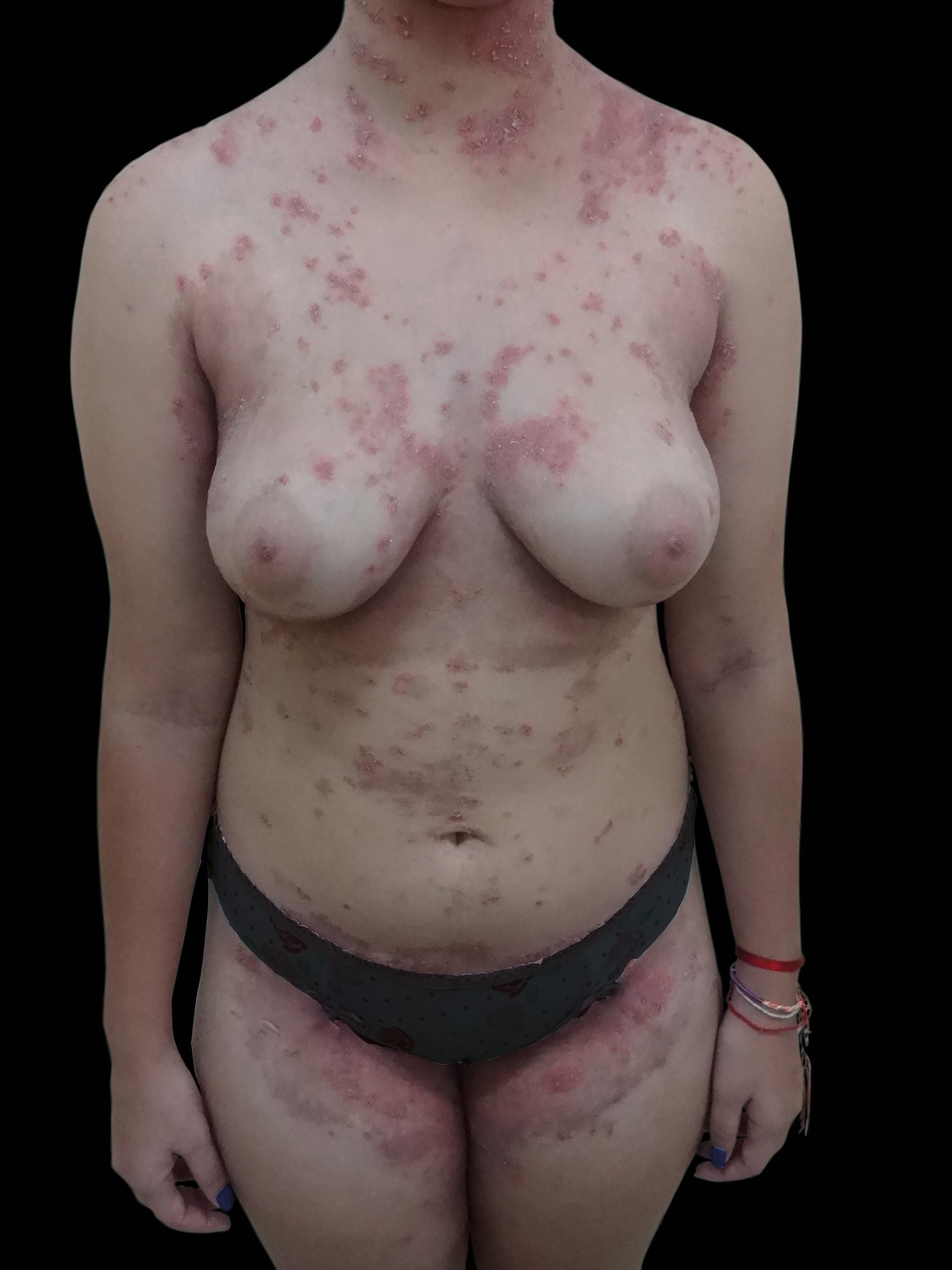
Case ReportA 15-year-old female patient presented with a 4-year history of pustular lesions on the proximal region of the upper limbs with a subsequent impairment of the chest and abdomen. During this period, the lesions showed temporary episodes of improvement that evolved with residual hyperchromic macules.
Physical examination revealed pustular lesions with a slightly erythematous base, isolated or grouped in a serpiginous pattern, on the trunk and proximal region of the upper and lower limbs. Hyperchromic macules were observed mingled with pustular lesions. There were no lesions on the face, palms, soles, and mucous membranes (Figures 1 and 2). A complete blood count, serum protein electrophoresis and liver, kidney and thyroid functions showed normal results.
Histopathology revealed a subcorneal spongiform pustule (Figure 3). Direct immunofluorescence of the perilesional skin for IgA, IgM, IgG and fibrinogen was negative, thus excluding IgA pemphigus.
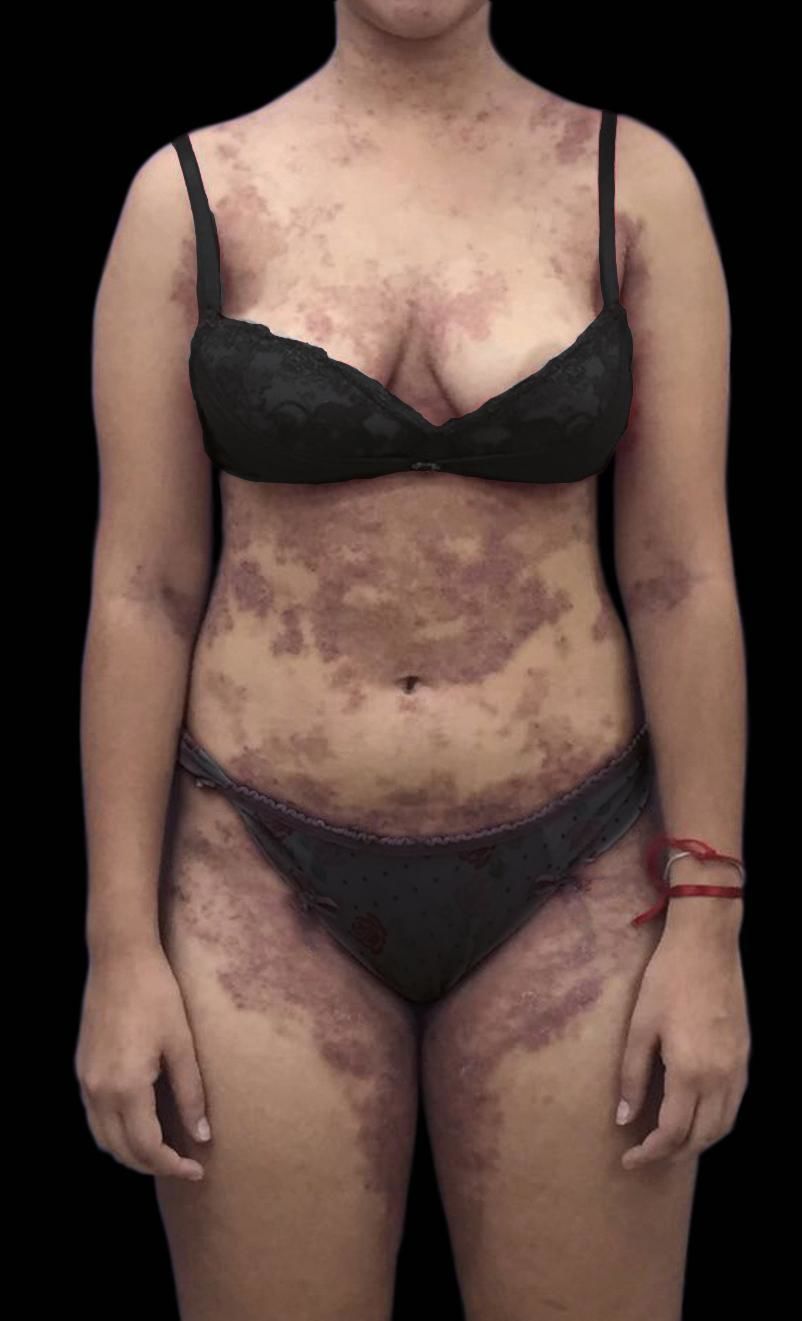
Considering the clinical and histopathological findings, we confirmed the diagnosis of SPD. The patient was treated with dapsone (100mg/day) with a significant improvement after one month, showing only hyperchromic macules and residual crusts (Figure 4). After treatment was instituted we observed a slight increase of total bilirubin (TB = 1.7, DB = 1.2) and a slight decrease of hemoglobin (Hb = 10.7). There were no alterations on the remaining markers of hemolysis and on G6PD dosage. We chose to maintain the treatment and throughout the patient’s follow-up all laboratory results remained stable. After complete clinical improvement, dapsone was tapered to 50mg/day and the patient was followed-up every three months. Nine months after treatment was started no flares had been noticed.
DiscussionSPD is a rare condition with few published case reports.. It was first described by Sneddon and Wilkinson in 1956 when the authors reported six cases of the disease, five of which in women between 40 and 68 years old.1 Since then, case reports have confirmed that this disease occurs more frequently in women of this age group, and few studies have reported the disease in children or teenagers.
The first case of pediatric SPD was reported in 1958 by Sakany et al. in a 10-year-old patient.3 Later in 1974, a case report of two children with a classic clinical presentation was published, however, only one child responded well to dapsone.4 Since then, the case reports of pediatric SPD have indicated that the clinical condition is similar both in children and adults. In 2013, Scalvenzi et al. reported a case of a 7-year-old patient with an erythematous-base pustular eruption for three weeks and described it as “half-and-half”, indicating that the lower half of the lesion presented a sterile purulent content and the upper half a serous one, which is characteristic of the disease.1 There are less than 20 cases of pediatric SPD described in the literature.5-10
Histopathology of SPD does not show pathognomonic findings; however, it does reveal subcorneal pustules and dermal infiltrate containing mainly neutrophils with few eosinophils, little or no spongiosis, and rarely acantholysis. Characteristically, direct immunofluorescence studies are negative. The differential diagnosis of SPD includes pustular psoriasis, IgA pemphigus, pemphigus foliaceus, dermatitis herpetiformis, impetigo, and acute generalized exanthematous pustulosis (AGEP).2
Despite the well documented association of SPD with multiple myeloma, IgA monoclonal gammopathy, chronic lymphocytic leukemia, thymoma, rheumatoid arthritis and thyroid disorders, our patient did not show any clinical symptom or laboratory result of systemic diseases during follow-up.
The first choice of treatment in children is dapsone 100-150mg/day, similar to what is indicated for adults. However, cases of hematologic toxicity to the drug have been reported in children, mainly hemolytic anemia and methemoglobinemia. In 2003, Koçak et al. observed a decrease in hemoglobin in a child with SPD treated with dapsone for three weeks, which normalized after the drug was withdrawn. In 2009, Tosi et al. published two cases of pediatric SPD in which one child manifested methemoglobinemia when treated with dapsone, which was also reversed once the drug was discontinued.6,9
Even though our patient showed a slight decrease in hemoglobin and a slight increase in bilirubin, her laboratory results and clinical evaluations remained stable throughout monthly follow-ups. Thus, there was no need to reduce the dosage of the drug before complete clinical improvement. Although dapsone remains the first choice of treatment, a rigorous clinical and laboratorial surveillance is needed in order to detect the first manifestations of its toxicity. Other therapeutic options for children are topical or systemic corticosteroids and acitretin.6,9
Financial support: none.
Conflict of interest: none.