Pemphigus vulgaris (PV) is a severe autoimmune blistering dermatosis. Genetic, malignant, and drug-induced PV triggers have been reported. Here we report two cases of patients who had severe aggravation or exacerbation of PV after COVID-19 vaccination.
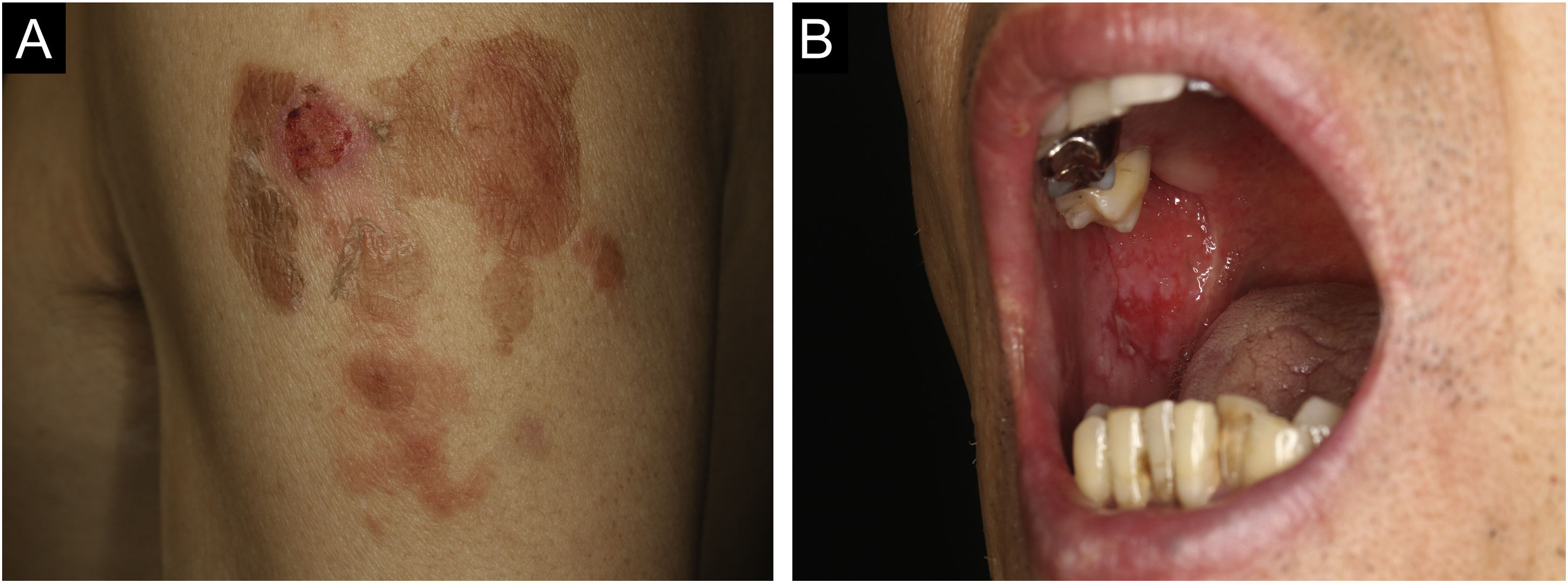
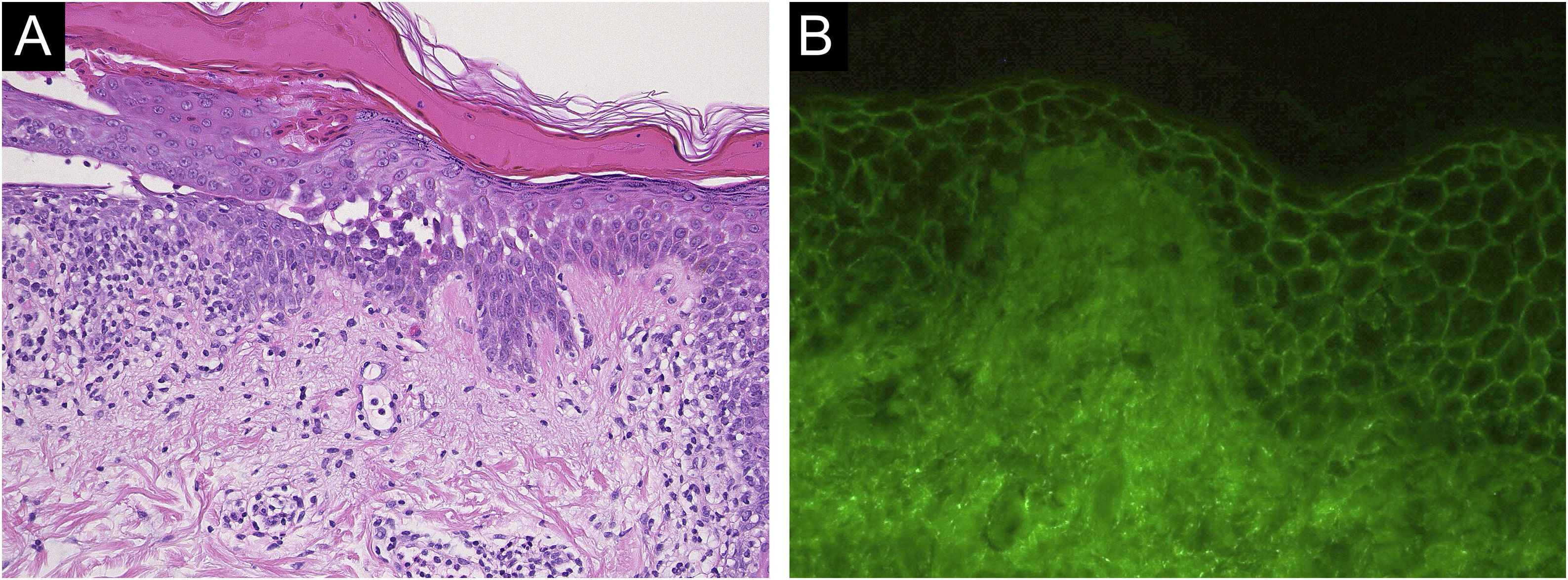
Case 1: A 60-year-old man with a two months history of painful erosion on the oral mucosa, which was treated by an otolaryngologist and an internist, received the second dose of the COVID-19 vaccine (Comirnaty®). One week later, erythema and erosions appeared at the vaccination site on his left arm. Subsequently, he developed erythema and erosions on his trunk and was referred to our department one month after the vaccination. Clinical examination showed the presence of post-bullous erosions, especially on the trunk, scalp, and left arm (Fig. 1A). He also had multiple erosions on the buccal mucosa (Fig. 1B). A skin biopsy showed acantholysis within the lower epidermal layers, and the presence of dense lymphocytic and eosinophilic dermal infiltrates (Fig. 2A). Direct immunofluorescence (DIF) revealed intercellular deposition of IgG and C3 in the epidermal cells (Fig. 2B). Serum levels of anti-Desmoglein (Dsg)-1 antibodies (120 U/mL, normal < 3 U/mL) and anti-Dsg-3 antibodies (262 U/mL, normal < 3 U/mL) were elevated. Oral prednisolone (50 mg/day (1 mg/kg/day)) was started; however, the response was poor and thus methylprednisolone pulse (1000 mg/day for consecutive three days), plasma exchange, and methotrexate (6 mg/week) were added. After obtaining remission, he received third and fourth dose of the COVID-19 vaccination without recurrence.
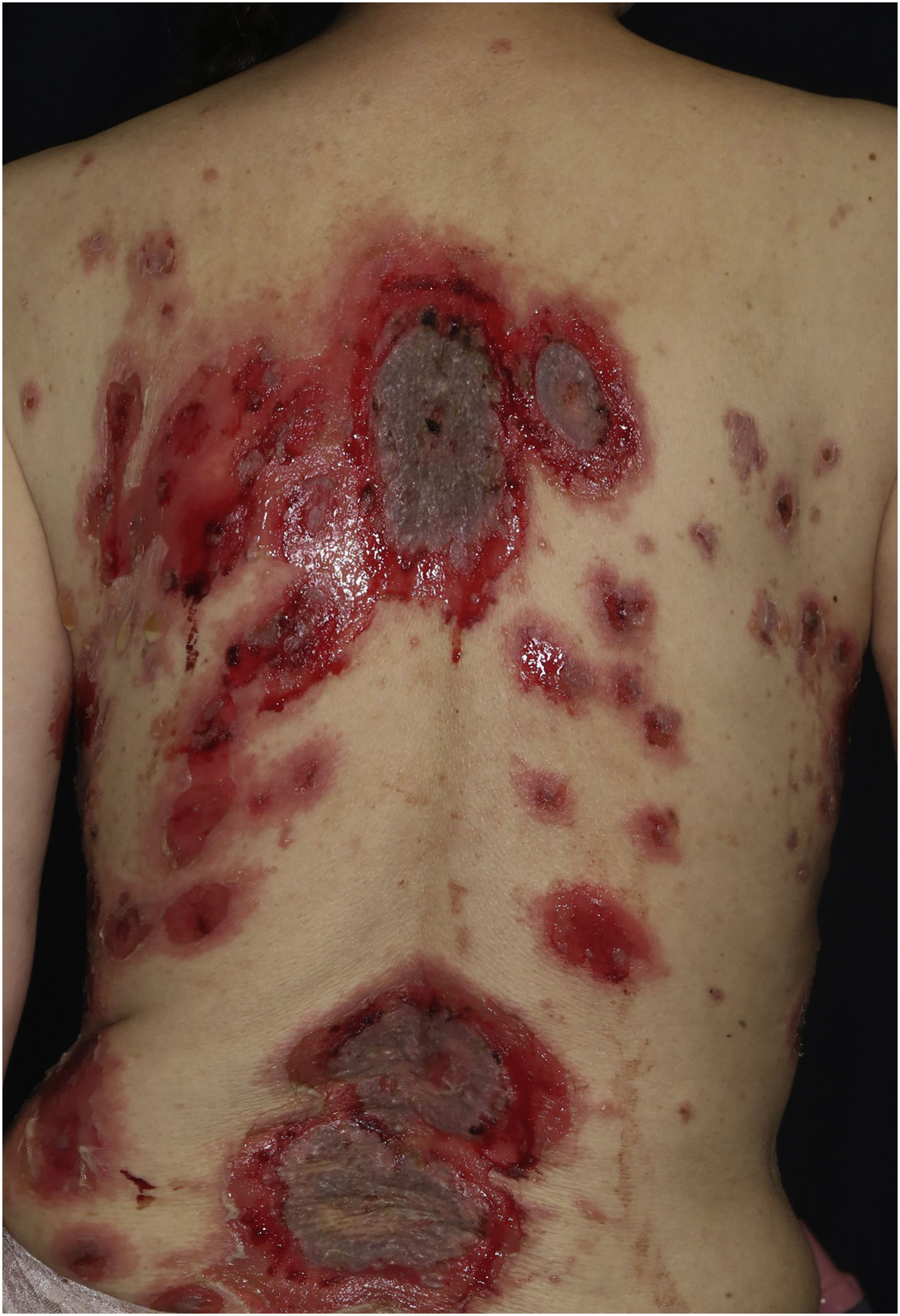
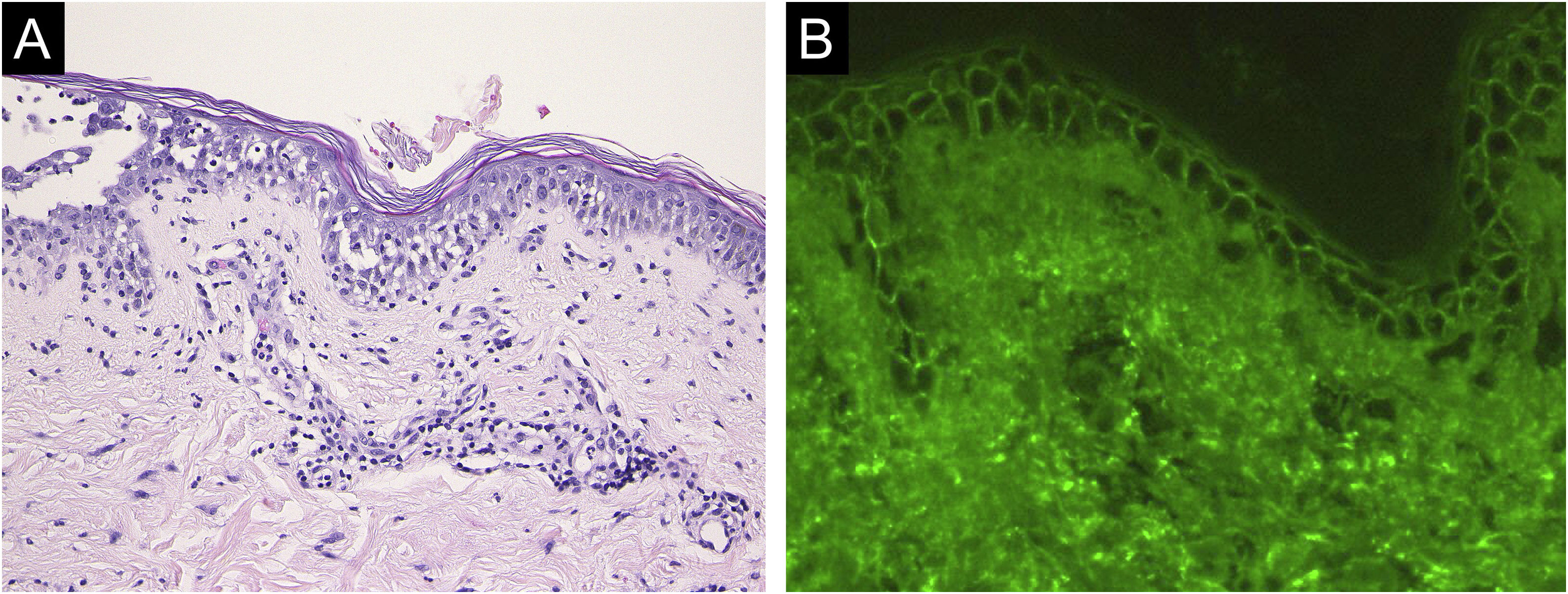
Case 2: A 69-year-old woman received the third dose of the COVID-19 vaccine (Spikevax®), and at about the same time, she experienced a scald injury on her right arm. Subsequently, she developed erosions on her extremities and trunk, which gradually increased in number, and was referred to our department three months after the vaccination. Clinical examination showed extensive erythema, flaccid blisters and post-bullous erosions on the trunk (Fig. 3). She also had multiple erosions on the oral mucosa. A skin biopsy from her abdomen revealed acantholysis within the lower epidermal layers, and the presence of dense lymphocytic and neutrophilic dermal infiltrates (Fig. 4A). DIF revealed intercellular deposition of IgG (Fig. 4B) and C3 in the lower epidermal cells. Serum levels of anti-Dsg-3 antibodies were high (8360 U/mL, normal < 3 U/mL), whereas those of anti-Dsg-1 antibodies were normal. Treatment with oral prednisolone (45 mg/day [1 mg/kg/day]), methylprednisolone pulse therapy (1000 mg/day for consecutive three days), and azathioprine (100 mg/day) resulted in complete epithelialization of erosions after 5 weeks of treatment.
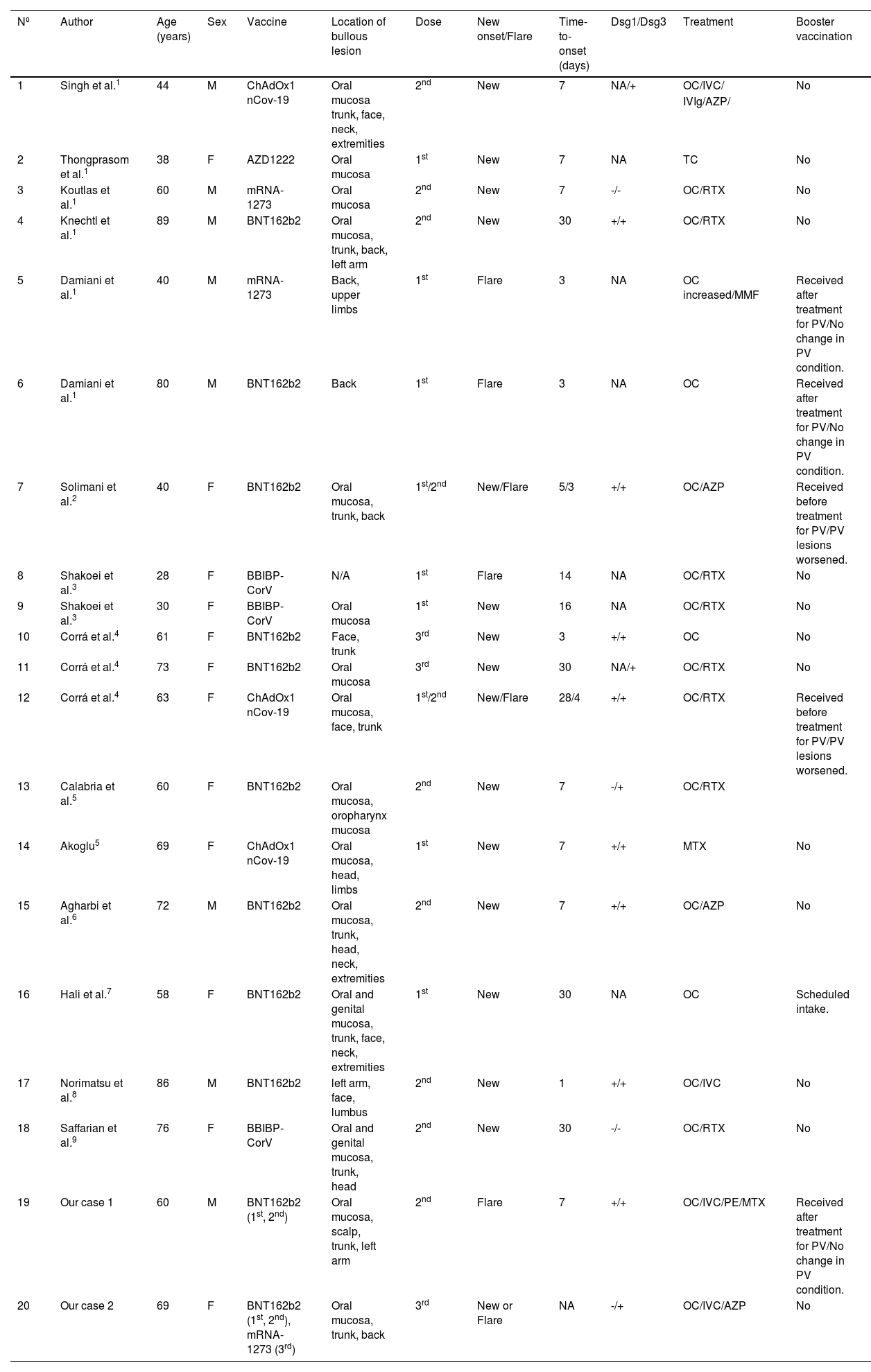
New onset or exacerbation of PV triggered by vaccinations or viral infections have been reported.1–9 There have been reported cases of PV induction or exacerbation following vaccination against influenza, rabies, hepatitis B, tetanus and diphtheria.1 In addition, cases of induction or exacerbation of autoimmune bullous diseases, psoriasis, lichen planus, dermatomyositis, and SLE, following COVID-19 vaccination have recently been reported.10 Activation of innate immunity due to the vaccine is thought to be the cause of exacerbation or development of skin symptoms. BNT162b2 injection induces activation of T-cells and B-cells, and after injection, CD4+ and CD8+ T-cells increase with production of IFN-γ and IL-2.2 It has been suggested that COVID-19 vaccination contributes to the production of cytokines like IL-4, IL-17, and IL-21 that play important roles in autoimmune bullous diseases such as PV.3 Vaccinations also activate B-cells, leading to increased antibody production.4 The reported cases1–9 of PV that developed de novo or deteriorated following COVID-19 vaccination are summarized in Table 1. PV developed a median of 7 (range 1–30) days each after the first, second, and third vaccinations. In contrast, the median time for cases of exacerbations was 3 days (range 3‒14), which is a significantly shorter period of time than the onset cases. However, there are two cases who were able to receive additional vaccinations after undergoing enhanced treatment for PV without flare-up of the disease. One of our cases also allowed for additional COVID-19 vaccinations without worsening the disease. The COVID-19 vaccine can certainly exacerbate PV in very rare cases, but even if exacerbation occurs, the vaccine can be safely administered in PV patients whose disease is well-controlled. Since vaccination is a necessary procedure to prevent aggravation of COVID-19 in immunosuppressed patients, the rare cases of progression of PV should not discourage the vaccination of patients with PV.
Reported cases of pemphigus vulgaris triggered by, or exacerbated following, COVID-19 vaccination.
| Nº | Author | Age (years) | Sex | Vaccine | Location of bullous lesion | Dose | New onset/Flare | Time-to-onset (days) | Dsg1/Dsg3 | Treatment | Booster vaccination |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Singh et al.1 | 44 | M | ChAdOx1 nCov-19 | Oral mucosa trunk, face, neck, extremities | 2nd | New | 7 | NA/+ | OC/IVC/ | No |
| IVIg/AZP/ | |||||||||||
| 2 | Thongprasom et al.1 | 38 | F | AZD1222 | Oral mucosa | 1st | New | 7 | NA | TC | No |
| 3 | Koutlas et al.1 | 60 | M | mRNA-1273 | Oral mucosa | 2nd | New | 7 | -/- | OC/RTX | No |
| 4 | Knechtl et al.1 | 89 | M | BNT162b2 | Oral mucosa, trunk, back, left arm | 2nd | New | 30 | +/+ | OC/RTX | No |
| 5 | Damiani et al.1 | 40 | M | mRNA-1273 | Back, upper limbs | 1st | Flare | 3 | NA | OC increased/MMF | Received after treatment for PV/No change in PV condition. |
| 6 | Damiani et al.1 | 80 | M | BNT162b2 | Back | 1st | Flare | 3 | NA | OC | Received after treatment for PV/No change in PV condition. |
| 7 | Solimani et al.2 | 40 | F | BNT162b2 | Oral mucosa, trunk, back | 1st/2nd | New/Flare | 5/3 | +/+ | OC/AZP | Received before treatment for PV/PV lesions worsened. |
| 8 | Shakoei et al.3 | 28 | F | BBIBP-CorV | N/A | 1st | Flare | 14 | NA | OC/RTX | No |
| 9 | Shakoei et al.3 | 30 | F | BBIBP-CorV | Oral mucosa | 1st | New | 16 | NA | OC/RTX | No |
| 10 | Corrá et al.4 | 61 | F | BNT162b2 | Face, trunk | 3rd | New | 3 | +/+ | OC | No |
| 11 | Corrá et al.4 | 73 | F | BNT162b2 | Oral mucosa | 3rd | New | 30 | NA/+ | OC/RTX | No |
| 12 | Corrá et al.4 | 63 | F | ChAdOx1 nCov-19 | Oral mucosa, face, trunk | 1st/2nd | New/Flare | 28/4 | +/+ | OC/RTX | Received before treatment for PV/PV lesions worsened. |
| 13 | Calabria et al.5 | 60 | F | BNT162b2 | Oral mucosa, oropharynx mucosa | 2nd | New | 7 | -/+ | OC/RTX | |
| 14 | Akoglu5 | 69 | F | ChAdOx1 nCov-19 | Oral mucosa, head, limbs | 1st | New | 7 | +/+ | MTX | No |
| 15 | Agharbi et al.6 | 72 | M | BNT162b2 | Oral mucosa, trunk, head, neck, extremities | 2nd | New | 7 | +/+ | OC/AZP | No |
| 16 | Hali et al.7 | 58 | F | BNT162b2 | Oral and genital mucosa, trunk, face, neck, extremities | 1st | New | 30 | NA | OC | Scheduled intake. |
| 17 | Norimatsu et al.8 | 86 | M | BNT162b2 | left arm, face, lumbus | 2nd | New | 1 | +/+ | OC/IVC | No |
| 18 | Saffarian et al.9 | 76 | F | BBIBP-CorV | Oral and genital mucosa, trunk, head | 2nd | New | 30 | -/- | OC/RTX | No |
| 19 | Our case 1 | 60 | M | BNT162b2 (1st, 2nd) | Oral mucosa, scalp, trunk, left arm | 2nd | Flare | 7 | +/+ | OC/IVC/PE/MTX | Received after treatment for PV/No change in PV condition. |
| 20 | Our case 2 | 69 | F | BNT162b2 (1st, 2nd), mRNA-1273 (3rd) | Oral mucosa, trunk, back | 3rd | New or Flare | NA | -/+ | OC/IVC/AZP | No |
NA, Noavailable; DSG1, Antibody anti-Desmoglein 1; DSG3, Antibody anti-Desmoglein 3; TC, Topical Corticosteroids; OC, Oral Corticosteroids; IVC, Intravenous Corticosteroids; RTX, Rituximab; MTX, Methotrexate; AZP, Azathioprine; MMF, Mycophenolate Mofetil; IVIg, Intravenous high-dose Immunoglobulin therapy; PE, Plasma Exchange; PV, Pemphigus Vulgaris.
None declared.
Authors’ contributionsKinuko Irie: Critical literature review; Data collection, analysis and interpretation; preparation and writing of the manuscript; statistical analysis; study conception and planning; approval of the final version of the manuscript.
Toshiyuki Yamamoto: Study conception and planning; manuscript critical review; approval of the final version of the manuscript.
Conflicts of interestNone declared.
Study conducted at the Fukushima Medical University, Fukushima, Japan.