There have been no studies to date on the frequency and reactivity of aanti-melanoma differentiation-associated gene 5 (anti-MDA-5) in samples from the Brazilian population with dermatomyositis.
Objectives.To analyze this autoantibody in the Brazilian population.
Methods:This was a single-center cross-sectional study in which 131 consecutive adult patients (109 dermatomyositis and 22 clinically amyopathic dermatomyositis) with active disease were evaluated from 2000 to 2016. Analysis of the anti-MDA-5 autoantibody was performed by ELISA.
Results:The presence of this autoantibody was observed in 14.7% and 22.7% of patients with dermatomyositis and clinically amyopathic dermatomyositis, respectively. In the case of dermatomyositis, the autoantibody was associated less frequently with Raynaud’s phenomenon and periungual hyperemia (P<0.05). In clinically amyopathic dermatomyositis, the presence of this autoantibody was not associated statistically with any demographic, clinical, laboratory, or imaging characteristics.
Study limitations:The cross-sectional study design did not allow establishing a temporal correlation between anti-MDA-5 autoantibody and various study variables. In addition, pulmonary function tests were not performed in the patients.
Conclusions.The frequency of anti-MDA-5 autoantibody was comparable to that of other populations with dermatomyositis, but with a different reactivity than described in the literature. In addition, there was a phenotypic variability between our patients with clinically amyopathic dermatomyositis and those described in the literature. Further studies are needed to confirm the current study’s findings and elucidate this autoantibody’s reactivity in Brazilians with idiopathic inflammatory myopathies.
Dermatomyositis (DM) is an autoimmune inflammatory myopathy characterized by muscle weakness of the limbs, mainly proximal, symmetric, and progressive, in addition to classic cutaneous alterations such as heliotrope rash and Gottron’s papules. It can also present other skin lesions such as cuticular hypertrophy, periungual hyperemia, photosensitivity, calcinosis, “V-neck sign”, and “shawl sign”, among others.1-3
In 10-15% of cases, DM patients do not present muscle involvement, thus characterizing the DM subset known as amyopathic or sine myositis.4,5 DM can also be classified as hypomyopathic, with altered laboratory results, evidencing some degree of myopathy. These two forms of DM (amyopathic and hypomyopathic) are called clinically amyopathic DM.2,5
Sato et al.6 identified the autoantibody to the 140-kDa clinically amyopathic dermatomyositis peptide (CADM-140) in Japanese patients with clinically amyopathic DM and whose principal characteristic was rapidly progressive pulmonary involvement. The target antigen for this autoantibody is the 140-kDa cytoplasmic protein called melanoma differentiation associated gene 5 (MDA-5), an RNA-specific helicase.7 Therefore, anti-CADM-140 is now also referred to as anti-MDA-5.7
The clinical characteristics and prognosis of patients with anti-MDA-5 autoantibodies are variable. The literature mentions the relationship between anti-MDA-5 and skin eruptions, rapid and progressive development of interstitial lung disease, skin lesions (rash, palmar papules, and/or ulcerations), joint and lung involvement, and/or worse prognosis in DM patients.7-13 Some of these patients also presented a similar phenotype to that of anti-synthetase syndrome.14 However, this wide phenotypical variability between the studies on the association with anti-MDA-5 autoantibody may be due to the heterogeneity of the populations studied, thus justifying the importance of assessing Brazilian patients with idiopathic inflammatory myopathies.
These studies also failed to mention possible confounders (smoking, chronic obstructive pulmonary disease, and overlapping of other systemic autoimmune diseases such as systemic sclerosis, systemic lupus erythematosus, and Sjögren’s syndrome, among others), which could interfere in the correlation between anti-MDA-5 autoantibody and the patients’ clinical and laboratory parameters.8,10-16
Presence of anti-MDA-5 autoantibody had not been analyzed previously in Brazilian patients with DM, thus justifying the current study, with the aim of verifying the autoantibody’s prevalence in patients with DM and clinically amyopathic DM, without possible confounders. The study also aimed to assess a possible association between the autoantibody and demographic, clinical, laboratory, and imaging parameters.
MethodsThis was a single-center cross-sectional study to evaluate the presence of anti-MDA-5 autoantibody in adult patients with DM, defined according to the criteria proposed by Bohan and Peter.1 Clinically amyopathic DM was determined according to the criteria of Gerami et al.16 All the assessed patients came from the same tertiary service, from 2000 to 2016.
The study was approved by the local Institutional Review Board (number 1.483.411).
The exclusion criteria were: patients with overlapping systemic autoimmune diseases, neoplasms, pulmonary infections (tuberculosis, aspergilloma), or chronic obstructive pulmonary disease; history of smoking; history of prior exposure to statins or fibrates; and suspected cases of muscular dystrophies.
Of the 218 patients that were initially admitted, 87 were excluded after application of the exclusion criteria. We thus analyzed 131 consecutive cases, of which 109 (83.2%) were DM and 22 (16.8%) clinically amyopathic DM.
The following data from the eligible patients were assessed, based on the electronic patient files, with pre-standardized and parameterized information, covering the data from the initial diagnostic workup and follow-up of patients with clinical and laboratory activity:
1. Demographics: current age; age at diagnosis of the disease; time between diagnosis and onset of symptoms; sex and race/color;
2. Clinical manifestations: constitutional symptoms (fever and weight loss); heliotrope rash; Gottron’s sign/papules; facial rash; “V-neck sign”; “shawl sign”; periungual hyperemia; vasculitis; calcinosis; ulcers; Raynaud’s phenomenon; mechanic’s hands; muscle weakness in the upper and lower limbs; Medical Research Council muscle strength grading; joint, gastrointestinal (high dysphagia), and pulmonary involvement (dyspnea: on moderate to small efforts; rapidly evolving dyspnea: in less than three months since onset of general symptoms);17
3. Altered pulmonary images obtained by CT: incipient lung disease, “ground glass” opacity, and bilateral basal pulmonary fibrosis;
4. Serum muscle enzyme levels in routine blood samples taken for medical consultation: creatinine phosphokinase (reference value: 32 – 294U/L) and aldolase (1.0 – 7.5U/L), performed with automated kinetic method.
Analysis of anti-MDA-5 autoantibody used serum samples in aliquots previously stored at -20°C, collected during the initial workup in patients with clinical and laboratory activity. The autoantibody was identified with enzyme-linked immunosorbent assay (ELISA), through the MDA-5 recombinant protein and anti-MDA-5 monoclonal antibody (MyBioSource, CA, USA). For the purposes of this study and to ensure positive results for anti-MDA-5, positivity was defined as patients with values more than three standard deviations above the mean in eight controls.
Statistical analysisThe Kolmogorov-Smirnov test was used to assess the distribution of each of the continuous variables. The results were presented as mean ±standard deviation for continuous variables and number (%) for categorical variables. Median values (interquartile range, 25% – 75%) were calculated for continuous variables that did not display normal distribution. Data on presence versus absence of anti-MDA-5 autoantibody were compared with Students t-test or Mann-Whitney test for continuous variables. Differences in categorical variables were calculated with Pearsons c2 or Fisher’s exact test. Statistical significance was set at p<0.05.
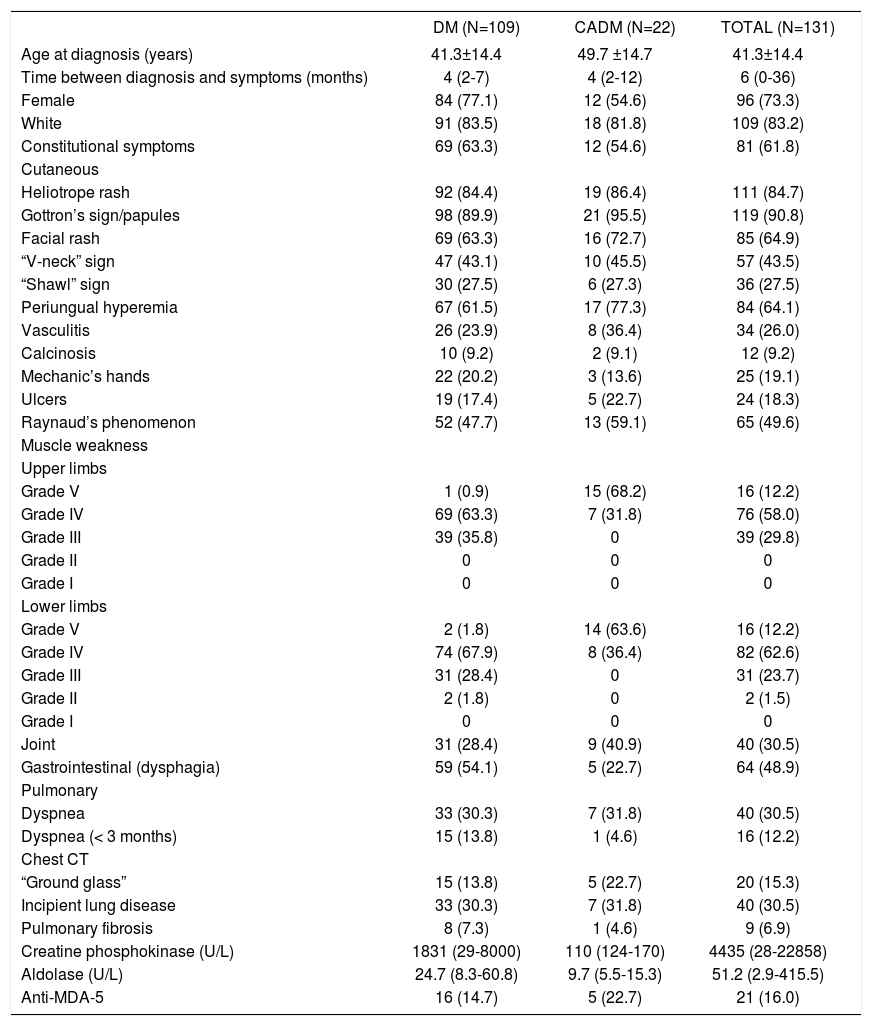
ResultsOf the 131 patients, 108 (83.2%) and 22 (16.8%) presented DM and clinically amyopathic DM, respectively. Table 1 shows these patients’ demographic, clinical, laboratory, and imaging data Age at diagnosis was 41.3 and 49.7 years, respectively, in patients with DM and clinically amyopathic DM. Female gender and white color predominated in both groups.
Demographic, clinical, laboratory, and imaging data of patients with dermatomyositis (classic and clinically amyopathic forms)
| DM (N=109) | CADM (N=22) | TOTAL (N=131) | |
|---|---|---|---|
| Age at diagnosis (years) | 41.3±14.4 | 49.7 ±14.7 | 41.3±14.4 |
| Time between diagnosis and symptoms (months) | 4 (2-7) | 4 (2-12) | 6 (0-36) |
| Female | 84 (77.1) | 12 (54.6) | 96 (73.3) |
| White | 91 (83.5) | 18 (81.8) | 109 (83.2) |
| Constitutional symptoms | 69 (63.3) | 12 (54.6) | 81 (61.8) |
| Cutaneous | |||
| Heliotrope rash | 92 (84.4) | 19 (86.4) | 111 (84.7) |
| Gottron’s sign/papules | 98 (89.9) | 21 (95.5) | 119 (90.8) |
| Facial rash | 69 (63.3) | 16 (72.7) | 85 (64.9) |
| “V-neck” sign | 47 (43.1) | 10 (45.5) | 57 (43.5) |
| “Shawl” sign | 30 (27.5) | 6 (27.3) | 36 (27.5) |
| Periungual hyperemia | 67 (61.5) | 17 (77.3) | 84 (64.1) |
| Vasculitis | 26 (23.9) | 8 (36.4) | 34 (26.0) |
| Calcinosis | 10 (9.2) | 2 (9.1) | 12 (9.2) |
| Mechanic’s hands | 22 (20.2) | 3 (13.6) | 25 (19.1) |
| Ulcers | 19 (17.4) | 5 (22.7) | 24 (18.3) |
| Raynaud’s phenomenon | 52 (47.7) | 13 (59.1) | 65 (49.6) |
| Muscle weakness | |||
| Upper limbs | |||
| Grade V | 1 (0.9) | 15 (68.2) | 16 (12.2) |
| Grade IV | 69 (63.3) | 7 (31.8) | 76 (58.0) |
| Grade III | 39 (35.8) | 0 | 39 (29.8) |
| Grade II | 0 | 0 | 0 |
| Grade I | 0 | 0 | 0 |
| Lower limbs | |||
| Grade V | 2 (1.8) | 14 (63.6) | 16 (12.2) |
| Grade IV | 74 (67.9) | 8 (36.4) | 82 (62.6) |
| Grade III | 31 (28.4) | 0 | 31 (23.7) |
| Grade II | 2 (1.8) | 0 | 2 (1.5) |
| Grade I | 0 | 0 | 0 |
| Joint | 31 (28.4) | 9 (40.9) | 40 (30.5) |
| Gastrointestinal (dysphagia) | 59 (54.1) | 5 (22.7) | 64 (48.9) |
| Pulmonary | |||
| Dyspnea | 33 (30.3) | 7 (31.8) | 40 (30.5) |
| Dyspnea (< 3 months) | 15 (13.8) | 1 (4.6) | 16 (12.2) |
| Chest CT | |||
| “Ground glass” | 15 (13.8) | 5 (22.7) | 20 (15.3) |
| Incipient lung disease | 33 (30.3) | 7 (31.8) | 40 (30.5) |
| Pulmonary fibrosis | 8 (7.3) | 1 (4.6) | 9 (6.9) |
| Creatine phosphokinase (U/L) | 1831 (29-8000) | 110 (124-170) | 4435 (28-22858) |
| Aldolase (U/L) | 24.7 (8.3-60.8) | 9.7 (5.5-15.3) | 51.2 (2.9-415.5) |
| Anti-MDA-5 | 16 (14.7) | 5 (22.7) | 21 (16.0) |
CADM: Clinically amyopathic dermatomyositis; MDA: melanoma differentiation-associated gene 5.
Data expressed as mean ± standard deviation, median (interquartile range 25% -75%), or percentage (%).
Constitutional symptoms were present in more than half of the cases in both groups (Table 1).
The principal cutaneous manifestations were heliotrope rash and Gottron’s sign/papules, followed by secondary lesions (facial rash, “V-neck” sign, “shawl” sign, periungual hyperemia, vasculitis, calcinosis, mechanic’s hand, ulcers, and Raynaud’s phenomenon).
As expected, muscle weakness in the limbs and elevated serum muscle enzymes were more evident in the classic form of DM, compared to clinically amyopathic DM.
Joint involvement and dysphagia were also found in both groups.
Dyspnea was reported in 30.3% and 31.8% of patients with DM and clinically amyopathic DM, respectively, while rapidly evolving dyspnea was present in 13.8% and 4.6% of the patients. In addition, the main CT finding was presence of incipient lung disease in both groups (one third of cases).
The anti-MDA-5 autoantibody was present in 14.7% of patients with DM and 22.7% of these with clinically amyopathic DM (Table 1).
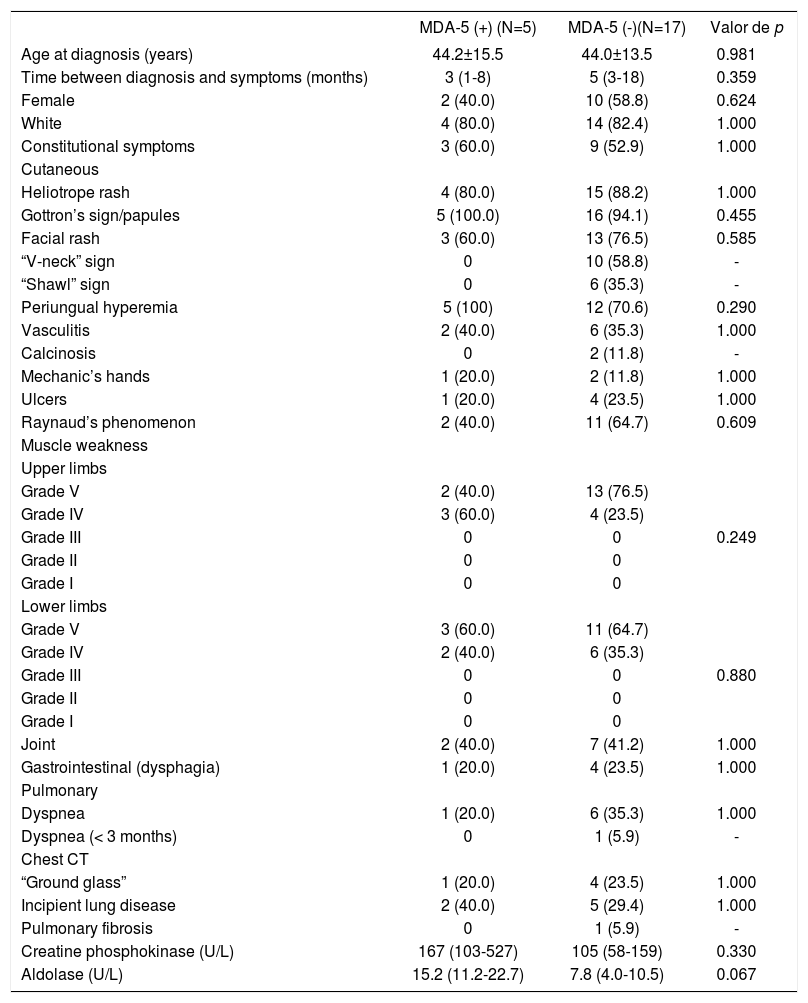
We also compared patients with DM (classic form) according to presence or absence of anti-MDA-5 autoantibody [MDA-5(+) vs. MDA-5(-)] (Table 2). Age, gender, race/color, and time between diagnosis and onset of simptoms were similar in the two groups. The presence of constitutional symptoms, clinical manifestations, and laboratory and imaging results were also comparable between the two groups, except for the lower frequency of periungual hyperemia (25% vs. 67.7%; p<0.002) and Raynaud’s phenomenon (18.8% vs. 52.7%; p<0.015) in anti-MDA-5(+) compared to anti-MDA-5 (-) patients.
Demographic, clinical, laboratory, and imaging data from patients with dermatomyositis (classic from) in relation to anti-MDA-5 autoantibody positivity
| MDA-5 (+) (N=16) | MDA-5 (-) (N=93) | p-value | |
|---|---|---|---|
| Age at diagnosis (years) | 41.8±14.0 | 40.5±14.5 | 0.749 |
| Time between diagnosis and symptoms (months) | 3 (1-5) | 4 (3-7) | 0.067 |
| Female | 13 (81.2) | 71 (76.3) | 1.000 |
| White | 15 (93.8) | 76 (81.7) | 1.000 |
| Constitutional symptoms | 11 (68.8) | 58 (62.4) | 0.782 |
| Cutaneous | |||
| Heliotrope rash | 13 (81.3) | 79 (85.0) | 0.713 |
| Gottron’s sign/papules | 14 (87.5) | 84 (90.3) | 0.663 |
| Facial rash | 11 (68.8) | 58 (62.4) | 0.781 |
| “V-neck” sign | 5 (31.3) | 42 (45.2) | 0.414 |
| “Shawl” sign | 1 (6.3) | 29 (31.2) | 0.065 |
| Periungual hyperemia | 4 (25.0) | 63 (67.7) | 0.002 |
| Vasculitis | 4 (25.0) | 22 (23.7) | 1.000 |
| Calcinosis | 1 (6.3) | 9 (9.7) | 1.000 |
| Mechanic’s hands | 1 (6.3) | 21 (22.6) | 0.185 |
| Ulcers | 4 (25.0) | 15 (16.1) | 0.474 |
| Raynaud’s phenomenon | 3 (18.8) | 49 (52.7) | 0.015 |
| Muscle weakness | |||
| Upper limbs | |||
| Grade V | 0 | 1 (1.1) | |
| Grade IV | 3 (18.8) | 66 (71.0) | |
| Grade III | 13 (81.2) | 26 (27.9) | 0.827 |
| Grade II | 0 | 0 | |
| Grade I | 0 | 0 | |
| Lower limbs | |||
| Grade V | 1 (6.3) | 1 (1.1) | |
| Grade IV | 10 (62.4) | 64 (68.7) | |
| Grade III | 5 (31.3) | 26 (28.0) | 0.733 |
| Grade II | 0 | 2 (2.2) | |
| Grade I | 0 | 0 | |
| Join | 5 (31.3) | 26 (28.0) | 0.771 |
| Gastrointestinal (dysphagia) | 6 (37.5) | 53 (57.0) | 0.180 |
| Pulmonary | |||
| Dyspnea | 4 (25.0) | 29 (31.2) | 0.772 |
| Dyspnea (< 3 months) | 2 (12.5) | 13 (14.0) | 1.000 |
| Chest CT | |||
| “Ground glass” | 1 (6.3) | 14 (15.1) | 0.693 |
| Incipient lung disease | 4 (25.0) | 29 (31.2) | 0.772 |
| Pulmonary fibrosis | 0 | 8 (8.6) | - |
| Creatine Phosphokinase (U/L) | 3726 (440-5884) | 1341 (340-8067) | 0.783 |
| Aldolase (U/L) | 39.6 (6.3-60.8) | 23.4 (8.4-68.9) | 0.975 |
MDA: melanoma differentiation-associated gene 5.
Data expressed as mean ± standard deviation, median (interquartile range 25% -75%), or percentage (%).
In patients with clinically amyopathic DM, all the demographic, clinical, laboratory, and imaging parameters were similar in those with MDA-5(+) and MDA-5(-) (Table 3).
Demographic, clinical, laboratory, and imaging data from patients with clinically amyopathic dermatomyositis in relation to anti-MDA-5 autoantibody positivity
| MDA-5 (+) (N=5) | MDA-5 (-)(N=17) | Valor de p | |
|---|---|---|---|
| Age at diagnosis (years) | 44.2±15.5 | 44.0±13.5 | 0.981 |
| Time between diagnosis and symptoms (months) | 3 (1-8) | 5 (3-18) | 0.359 |
| Female | 2 (40.0) | 10 (58.8) | 0.624 |
| White | 4 (80.0) | 14 (82.4) | 1.000 |
| Constitutional symptoms | 3 (60.0) | 9 (52.9) | 1.000 |
| Cutaneous | |||
| Heliotrope rash | 4 (80.0) | 15 (88.2) | 1.000 |
| Gottron’s sign/papules | 5 (100.0) | 16 (94.1) | 0.455 |
| Facial rash | 3 (60.0) | 13 (76.5) | 0.585 |
| “V-neck” sign | 0 | 10 (58.8) | - |
| “Shawl” sign | 0 | 6 (35.3) | - |
| Periungual hyperemia | 5 (100) | 12 (70.6) | 0.290 |
| Vasculitis | 2 (40.0) | 6 (35.3) | 1.000 |
| Calcinosis | 0 | 2 (11.8) | - |
| Mechanic’s hands | 1 (20.0) | 2 (11.8) | 1.000 |
| Ulcers | 1 (20.0) | 4 (23.5) | 1.000 |
| Raynaud’s phenomenon | 2 (40.0) | 11 (64.7) | 0.609 |
| Muscle weakness | |||
| Upper limbs | |||
| Grade V | 2 (40.0) | 13 (76.5) | |
| Grade IV | 3 (60.0) | 4 (23.5) | |
| Grade III | 0 | 0 | 0.249 |
| Grade II | 0 | 0 | |
| Grade I | 0 | 0 | |
| Lower limbs | |||
| Grade V | 3 (60.0) | 11 (64.7) | |
| Grade IV | 2 (40.0) | 6 (35.3) | |
| Grade III | 0 | 0 | 0.880 |
| Grade II | 0 | 0 | |
| Grade I | 0 | 0 | |
| Joint | 2 (40.0) | 7 (41.2) | 1.000 |
| Gastrointestinal (dysphagia) | 1 (20.0) | 4 (23.5) | 1.000 |
| Pulmonary | |||
| Dyspnea | 1 (20.0) | 6 (35.3) | 1.000 |
| Dyspnea (< 3 months) | 0 | 1 (5.9) | - |
| Chest CT | |||
| “Ground glass” | 1 (20.0) | 4 (23.5) | 1.000 |
| Incipient lung disease | 2 (40.0) | 5 (29.4) | 1.000 |
| Pulmonary fibrosis | 0 | 1 (5.9) | - |
| Creatine phosphokinase (U/L) | 167 (103-527) | 105 (58-159) | 0.330 |
| Aldolase (U/L) | 15.2 (11.2-22.7) | 7.8 (4.0-10.5) | 0.067 |
MDA: melanoma differentiation-associated gene 5.
Data expressed as mean ± standard deviation, median (interquartile range 25% -75%), or percentage (%).
The study showed that the frequency of anti-MDA-5 autoantibody in Brazilian patients with DM and clinically amyopathic DM was consistent with that reported in other populations, but with different reactivity from that described in the literature.
The study’s strengths include the evaluation of presence of anti-MDA-5 autoantibody in patients with a well-defined diagnosis of DM, based on the criteria proposed by Bohan and Peter1 and thus excluding cases with probable or possible diagnoses. For cases of clinically amyopathic DM, we used the updated definition proposed by Gerami et al.5 In addition, unlike previous studies in the literature, we applied rigorous exclusion criteria, for example patients with history of lung disease, tuberculosis, other systemic autoimmune diseases, neoplasms, smoking, and others, since such diseases, comorbidities, or habits could act as confounding factors for the interpretation and association between presence of anti-MDA-5 autoantibody and the clinical presentation of patients with DM or clinically amyopathic DM.
Although this was a retrospective study, the data had been previously standardized and parameterized and were thus trustworthy. In addition, in order to increase the specificity of the ELISA test, we applied a cutoff point of at least three standard deviations to include cases in the study, unlike previous studies such as Labrador-Horrillo et al.,14 who used two standard deviations as their cutoff.
According to the literature, positivity for anti-MDA-5 autoantibody can vary from 4.7 to 13.1% in DM and 10.0 to 18.8% in patients with clinically amyopathic DM.7,11-14 In the current study, MDA-5 autoantibody was present in 14.7% of patients with DM and 22.7% in clinically amyopathic DM, thus corroborating the rates reported in the literature. Since anti-MDA-5 autoantibody can vary according to disease activity, it is important to note that the current study only included patients with clinical and laboratory activity.18-20
In patients with classic DM, anti-MDA-5 autoantibody was associated with lower frequency of Raynaud’s phenomenon and periungual hyperemia. However, unlike other studies, we did not find an association between this autoantibody and the presence of skin ulcers, for example.12-14 Further studies are needed to better elucidate the relevance of these associations.
Neither did we observe an association between anti-MDA-5 autoantibody and a clinical presentation similar to anti-synthetase syndrome or its components (e.g., joint and pulmonary involvement). However, Hall et al.12 and Labrador-Horrillo et al.14 reported a high rate of anti-MDA-5 autoantibody in patients with symptoms suggestive of anti-synthetase syndrome, but without the presence of anti-synthetase autoantibody (e.g., anti-Jo-1 autoantibody). These discrepant data reinforce the presence of wide phenotypical variability among the different populations studied, as well as different anti-MDA-5 autoantibody reactivity according to the study population. Unlike our study, those authors did not mention possible confounding factors that could lead to lung or joint involvement in their series (e.g., overlapping diagnoses, history of smoking, chronic obstructive pulmonary disease (COPD), history of tuberculosis, etc.).12,14
In patients with clinically amyopathic DM we observed a variety of skin lesions, mainly Gottron’s sign/papules, heliotrope rash, facial rash, and periungual hyperemia. These were distributed the same way in patients with or without presence of anti-MDA-5 autoantibody. In contrast, studies in the literature reported a strong association between this autoantibody and the skin lesions found in patients with clinically amyopathic DM.6,8,12-14
Pulmonary involvement in patients with clinically amyopathic DM is relatively common.6,20-24 Particularly in Japanese and Chinese populations, it has been reported frequently in patients with rapidly progressive interstitial lung disease.6,20-24 Meanwhile, the prevalence of rapidly progressive interstitial lung disease in clinically amyopathic DM is relatively low in the United States, as in the current study.25
Previous studies also showed a strong association between anti-MDA-5 autoantibody and presence of pulmonary manifestations, which we did not observe in this study.6,9,14 One explanation is that we found a low rate of patients with pulmonary involvement and rapidly progressive dyspnea. These findings in turn may be the consequence of phenotypical diversity in the study sample, as well as the use of strict exclusion criteria, especially for factors related to pulmonary involvement (COPD, smoking, etc.).
As limitations, the study used a cross-sectional design, which prevented establishing a temporal correlation between anti-MDA-5 autoantibody and various independent variables. In addition, lung function tests were not performed in the patients.
ConclusionsAnti-MDA-5 autoantibody was highly prevalent in patients with DM and clinically amyopathic DM, consistent with findings in other populations, but with different reactivity than described in the literature. The patients in our sample also had different clinical characteristics from those reported in the literature, i.e., without major pulmonary and/or cutaneous involvement and with no similarity to anti-synthetase syndrome.
The study’s results can contribute to our understanding of different clinical, phenotypical, laboratory, and demographic characteristics of patients, thus allowing more individualized diagnosis and treatment.
Financial support: FAPESP #2015/12628-0 (IBPB) and #2014/09079-1 (SKS); CAPES (MGS).
Conflict of interest: None.