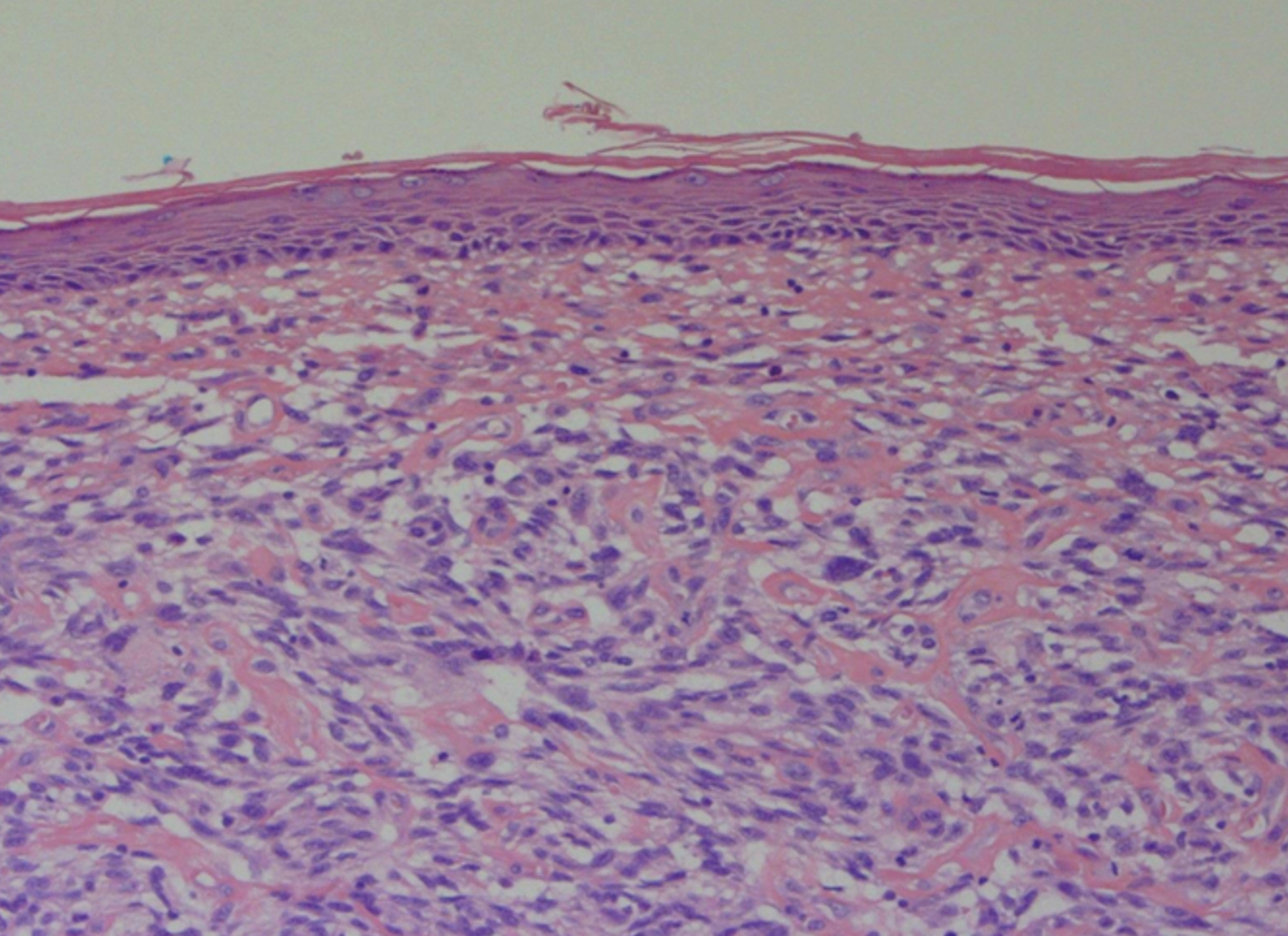
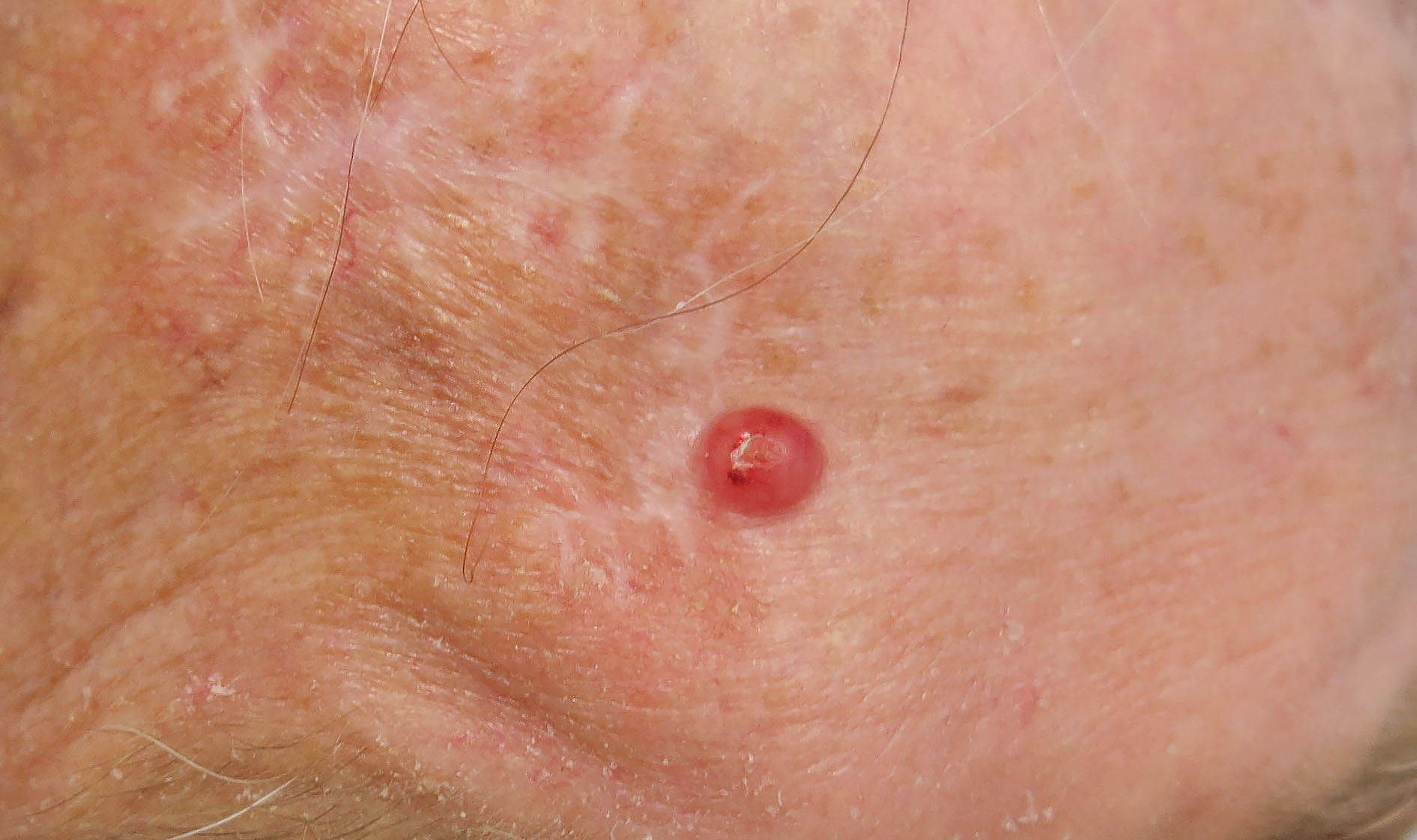
A 69-year-old Caucasian male was referred to our department with a 3-month history of an enlarging, asymptomatic nodule on his forehead. Examination revealed a 15mm, shiny, erythematous nodule with no associated lymphadenopathy. Further examination was unremarkable. Excision biopsy with a 4mm peripheral surgical margin revealed a dermal-based atypical spindle cell tumour extending from the dermo-epidermal junction to the deep subcutaneous adipose tissue. There was no evidence of tumour necrosis, lymphovascular invasion or perineural infiltration. No expression of cytokeratins, S100, desmin, or CD34 immunohistochemical markers was found, but there was positive expression of CD10. The histological margins were clear by 2mm peripherally and 1.2mm deep. Histopathological and immunohistochemical findings were consistent with the diagnosis of pleomorphic dermal sarcoma (Figure 1). The patient was followed up 3-monthly in a dedicated cutaneous sarcoma clinic. Six months after excision of the primary lesion, a new 5mm papule appeared on the excision scar (Figure 2). Repeat excision revealed recurrent pleomorphic dermal sarcoma with clear margins. The multidisciplinary team consensus was to treat this further with local adjuvant radiotherapy following a staging CT scan. At 7 months, staging CT revealed a new discrete nodule in the left lower lobe of the lung. CT-guided biopsy confirmed a CD10 positive spindle cell tumour consistent with metastatic pleomorphic dermal sarcoma. The patient subsequently died from pneumonia at 11 months following diagnosis, having been too unwell for palliative chemotherapy.
Pleomorphic dermal sarcoma (PDS), previously known as superficial malignant fibrous histiocytoma, or undifferentiated pleomorphic sarcoma of skin, is a rare dermal-based spindle cell tumour that may recur locally or metastasise.1 The term pleomorphic dermal sarcoma was introduced to reclassify atypical fibroxanthoma (AFX)-like tumours that have additional high-risk features suggestive of a greater malignant potential.2 Diagnostic differentiation from AFX requires the presence of one or more of: deep adipose tissue invasion, tumour necrosis, lymphovascular invasion, and/or perineural invasion.2 Clinically, PDS is indistinguishable from AFX and appears to form part of a biological continuum, with PDS having a significantly higher rate of local recurrence and metastatic spread.1,3,4 The first reported case series of PDS reviewed 32 cases and found tumour necrosis in 53%, lymphovascular invasion in 26%, and perineural infiltration in 29%, with CD10 expressed in all cases tested and a metastatic rate of 10%.3 The second reported series of 18 patients with PDS found that tumours arose mainly on the scalp and face of elderly patients (median age 81).1 Consistent histopathological features included spindle cells arranged in a fascicular pattern, and the presence of epithelioid and multinucleate giant cells with pleomorphic vesicular nuclei. Lesions in all patients demonstrated invasion to the subcutis with additional necrosis identified in 3 patients. Follow-up data revealed that 20% developed local recurrence and 20% distant metastases to the skin, lymph nodes, and/or lungs.1
Due to the predominant tumour location on the head and neck, complete histological clearance of the primary or recurrent tumour may be unachievable. Treatment with adjuvant radiotherapy may then be considered.4 In large defects the initiation of adjuvant radiotherapy tends to be delayed whilst waiting for wound healing.5 Müller et al. achieved successful graft healing without complications by treating a patient with an innovative technique where adjuvant radiotherapy was started immediately following excision and reconstruction performed subsequently.5
Our report highlights the risk of local recurrence and metastatic spread in this rare cutaneous tumour. Recognition of this greater malignant potential emphasises the need for evidence-based guidelines for the treatment of pleomorphic dermal sarcoma.
Financial support: None.
Conflict of interest: None.