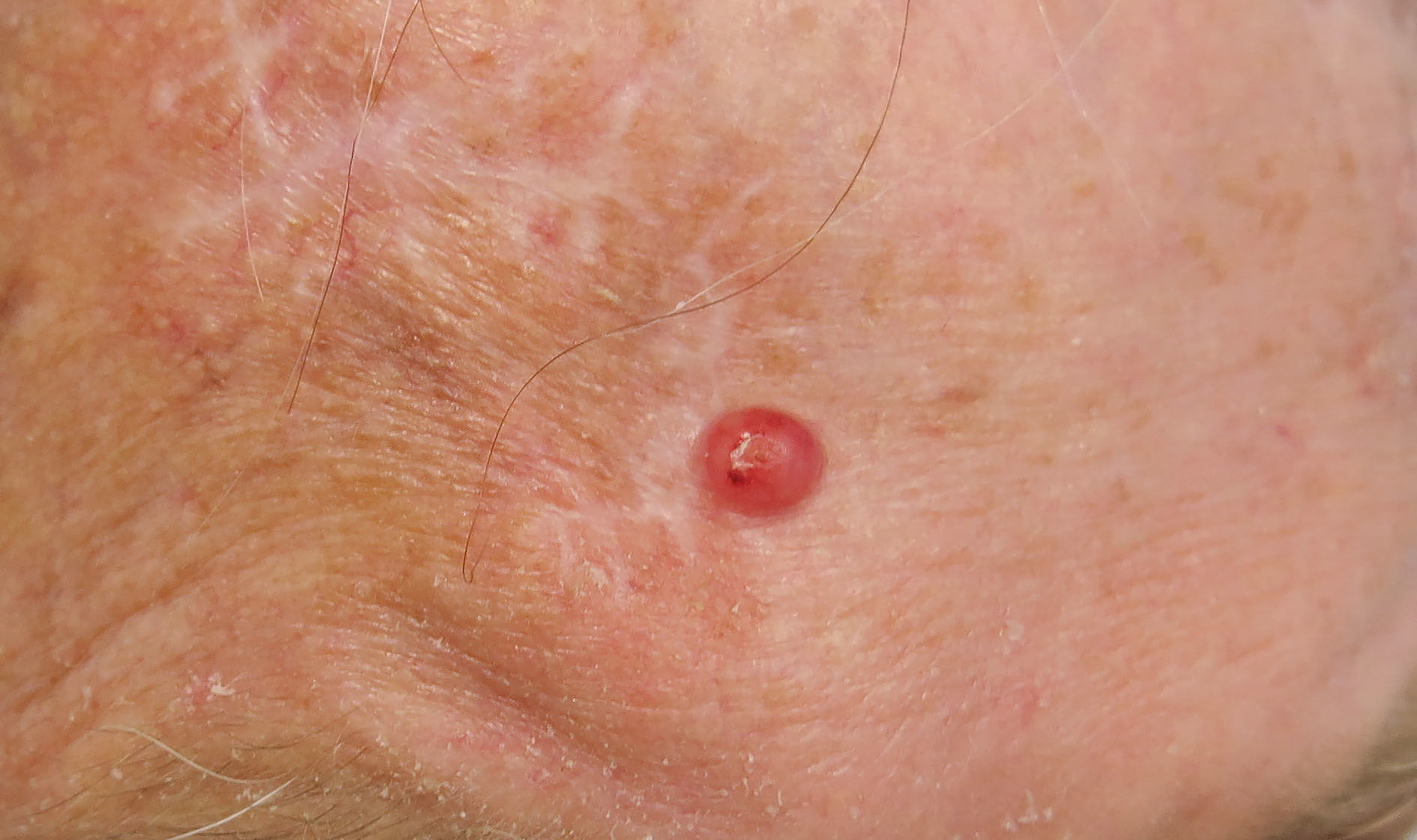
Dear editor,
One of the most frequent diagnoses in a dermatologist’s daily practice is viral warts. In many cases, the lesion is self-limiting and frequently resolves without therapeutic intervention – especially in recent cases – with little hyperkeratosis.1 However, periungual warts, in particular, are frequently associated with high recurrence rates, which poses a therapeutic challenge. Several therapeutic modalities are described in the literature. Some of them include surgical techniques that are sometimes painful and can lead to nail dystrophy. In such cases, physicians can resort to drugs used for immunotherapy with good resolution rates and aesthetic results. In this context, diphencyprone has been studied for some time, with controversial results.2 We describe the case of a female patient with difficult-to-treat periungual wart who showed considerable clinical signs of improvement after the use of diphencyprone.
A 13-year-old female patient presented with multiple periungual warts on nine fingers with one year of evolution (Figure 1). She had undergone previous treatment with lactic acid associated with salicylic acid without improvement. Due to the exuberance of the clinical picture, we decided to perform immunotherapy with a 2% solution of diphencyprone on the back skin. The patient returned after two weeks reporting application site reaction (blisters) and a slight improvement of the periungual lesions. After that, we initiated a therapy with a 0.1% solution of diphencyprone applied to the warts, always followed by occlusion for eight hours. The patient evolved with significant improvement of the lesions after three applications in biweekly intervals (Figure 2).
Immunotherapy consists of modulating the immune system, either to activate or to suppress immune responses. Its activation is especially important in the treatment of viral infections. For some time, immunotherapy has been studied in dermatology, especially in the treatment of recalcitrant viral warts, which includes topical, intralesional, or systemic treatments. It is theorized that the application of topical immunomodulators induces delayed-type or type IV hypersensitivity reactions. As a consequence, Th1 cells release cytokines that cause the recruitment of macrophages and natural killer cells that induce the death of infected keratinocytes.2 This reaction would justify the reduction of warts after sensitization on the back skin, even without direct application in the periungual region.
Among the topical immunomodulators, diphencyprone is one of the most studied with varying efficacy results revealed by the studies. Cure rates of treatment with diphencyprone vary from 44-88%. In a controlled open study of 170 patients with multiple viral warts (511 lesions), complete resolution of the lesions was seen in 141 patients (82.9%) and in 434 warts (84.8%). Younger patients (<20 years) and localized warts on the hands showed better results.3 To date, there are no randomized clinical trials comparing diphencyprone with other therapeutic modalities. Choi MH et al., in a non-randomized study of 147 patients, showed a statistical superiority of diphencyprone (93%) after 12 months of follow-up compared to cryotherapy (76%) (p > 0.05%). These results strengthen the hypothesis that diphencyprone stimulates direct immunity response against HPV.4 A series of cases specifically made with patients with periungual warts was conducted with 27 patients (66 lesions). Of them, 85% of patients (91% of the warts) achieved complete clearance. Warts located in the proximal nail fold showed a 95% higher success rate, compared to 87% and 86% for lateral fold and hyponychium, respectively.5
Although diphencyprone finds a theoretical basis in the international literature for the treatment of periungual and recalcitrant viral warts, we found no reports or discussions in the Brazilian literature. Our patient experienced previous therapeutic failure, showing excellent clinical results with diphencyprone. We call attention to this effective and safe drug that is rarely considered as an alternative in the therapeutic arsenal of the dermatologist.
Financial support: None.
Conflict of interests: None.