Tradicionalmente, o tratamento da psoríase depende do emprego de medicamentos tópicos e medicamentos clássicos, como metotrexato, acitretina ou ciclosporina, com resultados variados e numerosos efeitos colaterais. No entanto, nos últimos anos, o surgimento de terapias biológicas e de pequenas moléculas revolucionou seu tratamento.1 Dentre os agentes biológicos, os inibidores da interleucina (IL)‐23 desempenham papel fundamental na patogênese da psoríase.2 Atualmente, há três medicamentos anti‐IL‐23 aprovados: risanquizumabe, tildraquizumabe e guselcumabe. A quantidade de estudos que avaliam esses medicamentos na prática clínica para a psoríase é limitada. Ainda mais escassos são os estudos comparativos entre as três alternativas.
Este artigo apresenta os resultados de um estudo retrospectivo unicêntrico envolvendo todos os pacientes com psoríase tratados com medicamentos anti‐IL‐23. O objetivo era descrever a resposta em termos de eficácia e segurança do risanquizumabe, tildraquizumabe e guselcumabe na prática clínica. Além disso, objetivou‐se comparar a resposta entre os três medicamentos para detectar diferenças nos resultados obtidos com as três alternativas.
Pacientes com psoríase tratados com medicamentos anti‐IL‐23 em hospital terciário entre 2015 e 2020 foram avaliados retrospectivamente, monitorados por um ano. Os resultados obtidos em 16, 24 e 48‐52 semanas foram registrados em relação à Área de Psoríase e Índice de Gravidade (PASI, do inglês Psoriasis Area and Severity Index), ASC (área de superfície corporal), Avaliação Global do Investigador (IGA, do inglês Investigator's Global Assessment) e Índice de Qualidade de Vida em Dermatologia (DLQI, do inglês Dermatology Life Quality Index), bem como os eventos adversos relatados. Foram excluídos pacientes com menos de 16 semanas de seguimento, aqueles provenientes de ensaio clínico ou aqueles em que pelo menos 50% das variáveis registradas no estudo não estavam disponíveis.
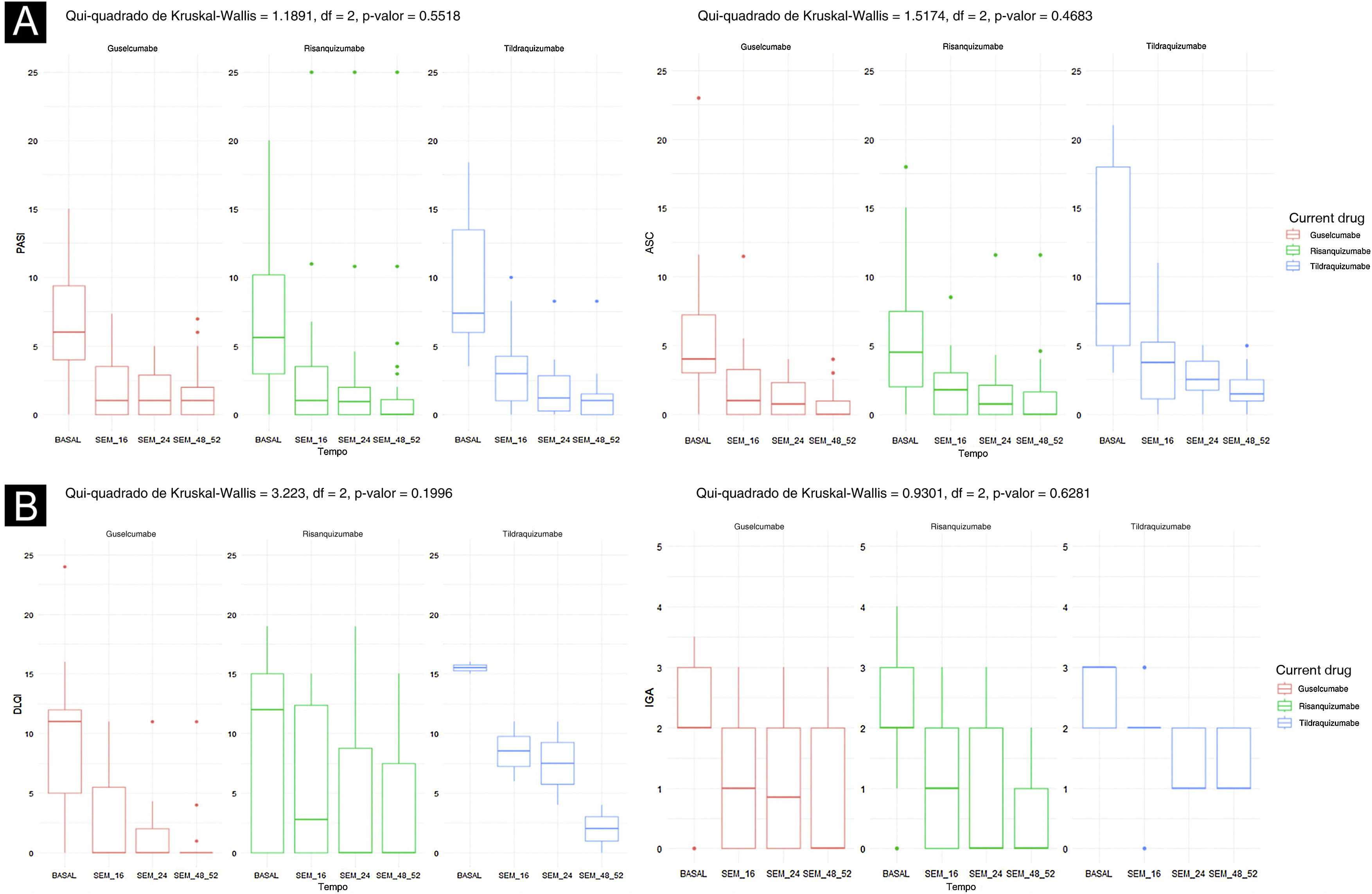
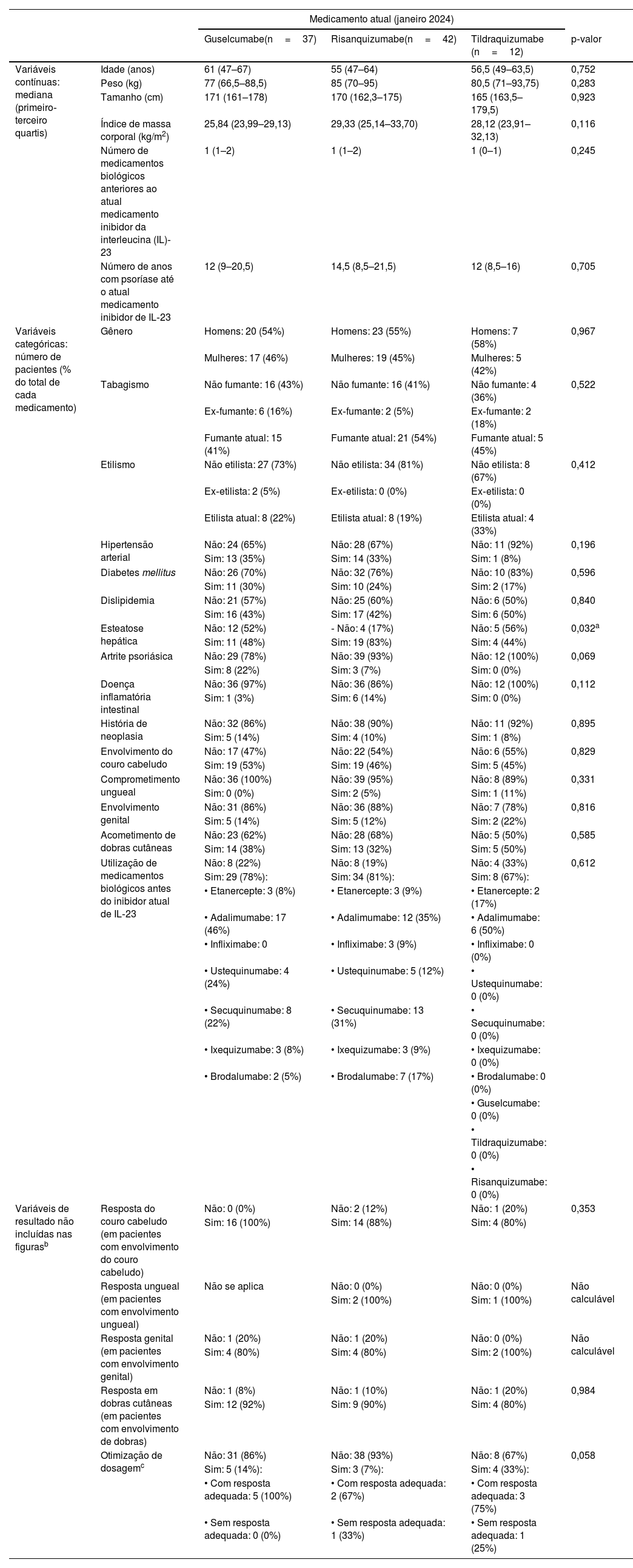
Foram incluídos 91 pacientes, dos quais 42 receberam risanquizumabe, 37 guselcumabe e 12 tildraquizumabe. A tabela 1 mostra as características basais dos três grupos, bem como algumas avaliações de resultados não incluídas na figura 1, que ilustra a evolução da resposta em termos de PASI, ASC, IGA e DLQI. Os três grupos foram comparáveis em relação às características basais e à gravidade clínica inicial da doença. A maioria dos pacientes era do gênero masculino, com idade em torno de 60 anos e com excesso de peso. Aproximadamente metade e um terço dos pacientes eram fumantes ou ex‐fumantes e etilistas ou ex‐etilistas, respectivamente. Onze por cento tinham artrite psoriásica em seguimento reumatológico, 10% tinham história de neoplasia e 7% apresentavam doença inflamatória intestinal.
Descrição da amostra e variáveis de desfecho não incluídas na figura 1
| Medicamento atual (janeiro 2024) | |||||
|---|---|---|---|---|---|
| Guselcumabe(n=37) | Risanquizumabe(n=42) | Tildraquizumabe (n=12) | p‐valor | ||
| Variáveis contínuas: mediana (primeiro‐terceiro quartis) | Idade (anos) | 61 (47–67) | 55 (47–64) | 56,5 (49–63,5) | 0,752 |
| Peso (kg) | 77 (66,5–88,5) | 85 (70–95) | 80,5 (71–93,75) | 0,283 | |
| Tamanho (cm) | 171 (161–178) | 170 (162,3–175) | 165 (163,5–179,5) | 0,923 | |
| Índice de massa corporal (kg/m2) | 25,84 (23,99–29,13) | 29,33 (25,14–33,70) | 28,12 (23,91–32,13) | 0,116 | |
| Número de medicamentos biológicos anteriores ao atual medicamento inibidor da interleucina (IL)‐23 | 1 (1–2) | 1 (1–2) | 1 (0–1) | 0,245 | |
| Número de anos com psoríase até o atual medicamento inibidor de IL‐23 | 12 (9–20,5) | 14,5 (8,5–21,5) | 12 (8,5–16) | 0,705 | |
| Variáveis categóricas: número de pacientes (% do total de cada medicamento) | Gênero | Homens: 20 (54%) | Homens: 23 (55%) | Homens: 7 (58%) | 0,967 |
| Mulheres: 17 (46%) | Mulheres: 19 (45%) | Mulheres: 5 (42%) | |||
| Tabagismo | Não fumante: 16 (43%) | Não fumante: 16 (41%) | Não fumante: 4 (36%) | 0,522 | |
| Ex‐fumante: 6 (16%) | Ex‐fumante: 2 (5%) | Ex‐fumante: 2 (18%) | |||
| Fumante atual: 15 (41%) | Fumante atual: 21 (54%) | Fumante atual: 5 (45%) | |||
| Etilismo | Não etilista: 27 (73%) | Não etilista: 34 (81%) | Não etilista: 8 (67%) | 0,412 | |
| Ex‐etilista: 2 (5%) | Ex‐etilista: 0 (0%) | Ex‐etilista: 0 (0%) | |||
| Etilista atual: 8 (22%) | Etilista atual: 8 (19%) | Etilista atual: 4 (33%) | |||
| Hipertensão arterial | Não: 24 (65%) | Não: 28 (67%) | Não: 11 (92%) | 0,196 | |
| Sim: 13 (35%) | Sim: 14 (33%) | Sim: 1 (8%) | |||
| Diabetes mellitus | Não: 26 (70%) | Não: 32 (76%) | Não: 10 (83%) | 0,596 | |
| Sim: 11 (30%) | Sim: 10 (24%) | Sim: 2 (17%) | |||
| Dislipidemia | Não: 21 (57%) | Não: 25 (60%) | Não: 6 (50%) | 0,840 | |
| Sim: 16 (43%) | Sim: 17 (42%) | Sim: 6 (50%) | |||
| Esteatose hepática | Não: 12 (52%) | ‐ Não: 4 (17%) | Não: 5 (56%) | 0,032a | |
| Sim: 11 (48%) | Sim: 19 (83%) | Sim: 4 (44%) | |||
| Artrite psoriásica | Não: 29 (78%) | Não: 39 (93%) | Não: 12 (100%) | 0,069 | |
| Sim: 8 (22%) | Sim: 3 (7%) | Sim: 0 (0%) | |||
| Doença inflamatória intestinal | Não: 36 (97%) | Não: 36 (86%) | Não: 12 (100%) | 0,112 | |
| Sim: 1 (3%) | Sim: 6 (14%) | Sim: 0 (0%) | |||
| História de neoplasia | Não: 32 (86%) | Não: 38 (90%) | Não: 11 (92%) | 0,895 | |
| Sim: 5 (14%) | Sim: 4 (10%) | Sim: 1 (8%) | |||
| Envolvimento do couro cabeludo | Não: 17 (47%) | Não: 22 (54%) | Não: 6 (55%) | 0,829 | |
| Sim: 19 (53%) | Sim: 19 (46%) | Sim: 5 (45%) | |||
| Comprometimento ungueal | Não: 36 (100%) | Não: 39 (95%) | Não: 8 (89%) | 0,331 | |
| Sim: 0 (0%) | Sim: 2 (5%) | Sim: 1 (11%) | |||
| Envolvimento genital | Não: 31 (86%) | Não: 36 (88%) | Não: 7 (78%) | 0,816 | |
| Sim: 5 (14%) | Sim: 5 (12%) | Sim: 2 (22%) | |||
| Acometimento de dobras cutâneas | Não: 23 (62%) | Não: 28 (68%) | Não: 5 (50%) | 0,585 | |
| Sim: 14 (38%) | Sim: 13 (32%) | Sim: 5 (50%) | |||
| Utilização de medicamentos biológicos antes do inibidor atual de IL‐23 | Não: 8 (22%) | Não: 8 (19%) | Não: 4 (33%) | 0,612 | |
| Sim: 29 (78%): | Sim: 34 (81%): | Sim: 8 (67%): | |||
| • Etanercepte: 3 (8%) | • Etanercepte: 3 (9%) | • Etanercepte: 2 (17%) | |||
| • Adalimumabe: 17 (46%) | • Adalimumabe: 12 (35%) | • Adalimumabe: 6 (50%) | |||
| • Infliximabe: 0 | • Infliximabe: 3 (9%) | • Infliximabe: 0 (0%) | |||
| • Ustequinumabe: 4 (24%) | • Ustequinumabe: 5 (12%) | • Ustequinumabe: 0 (0%) | |||
| • Secuquinumabe: 8 (22%) | • Secuquinumabe: 13 (31%) | • Secuquinumabe: 0 (0%) | |||
| • Ixequizumabe: 3 (8%) | • Ixequizumabe: 3 (9%) | • Ixequizumabe: 0 (0%) | |||
| • Brodalumabe: 2 (5%) | • Brodalumabe: 7 (17%) | • Brodalumabe: 0 (0%) | |||
| • Guselcumabe: 0 (0%) | |||||
| • Tildraquizumabe: 0 (0%) | |||||
| • Risanquizumabe: 0 (0%) | |||||
| Variáveis de resultado não incluídas nas figurasb | Resposta do couro cabeludo (em pacientes com envolvimento do couro cabeludo) | Não: 0 (0%) | Não: 2 (12%) | Não: 1 (20%) | 0,353 |
| Sim: 16 (100%) | Sim: 14 (88%) | Sim: 4 (80%) | |||
| Resposta ungueal (em pacientes com envolvimento ungueal) | Não se aplica | Não: 0 (0%) | Não: 0 (0%) | Não calculável | |
| Sim: 2 (100%) | Sim: 1 (100%) | ||||
| Resposta genital (em pacientes com envolvimento genital) | Não: 1 (20%) | Não: 1 (20%) | Não: 0 (0%) | Não calculável | |
| Sim: 4 (80%) | Sim: 4 (80%) | Sim: 2 (100%) | |||
| Resposta em dobras cutâneas (em pacientes com envolvimento de dobras) | Não: 1 (8%) | Não: 1 (10%) | Não: 1 (20%) | 0,984 | |
| Sim: 12 (92%) | Sim: 9 (90%) | Sim: 4 (80%) | |||
| Otimização de dosagemc | Não: 31 (86%) | Não: 38 (93%) | Não: 8 (67%) | 0,058 | |
| Sim: 5 (14%): | Sim: 3 (7%): | Sim: 4 (33%): | |||
| • Com resposta adequada: 5 (100%) | • Com resposta adequada: 2 (67%) | • Com resposta adequada: 3 (75%) | |||
| • Sem resposta adequada: 0 (0%) | • Sem resposta adequada: 1 (33%) | • Sem resposta adequada: 1 (25%) | |||
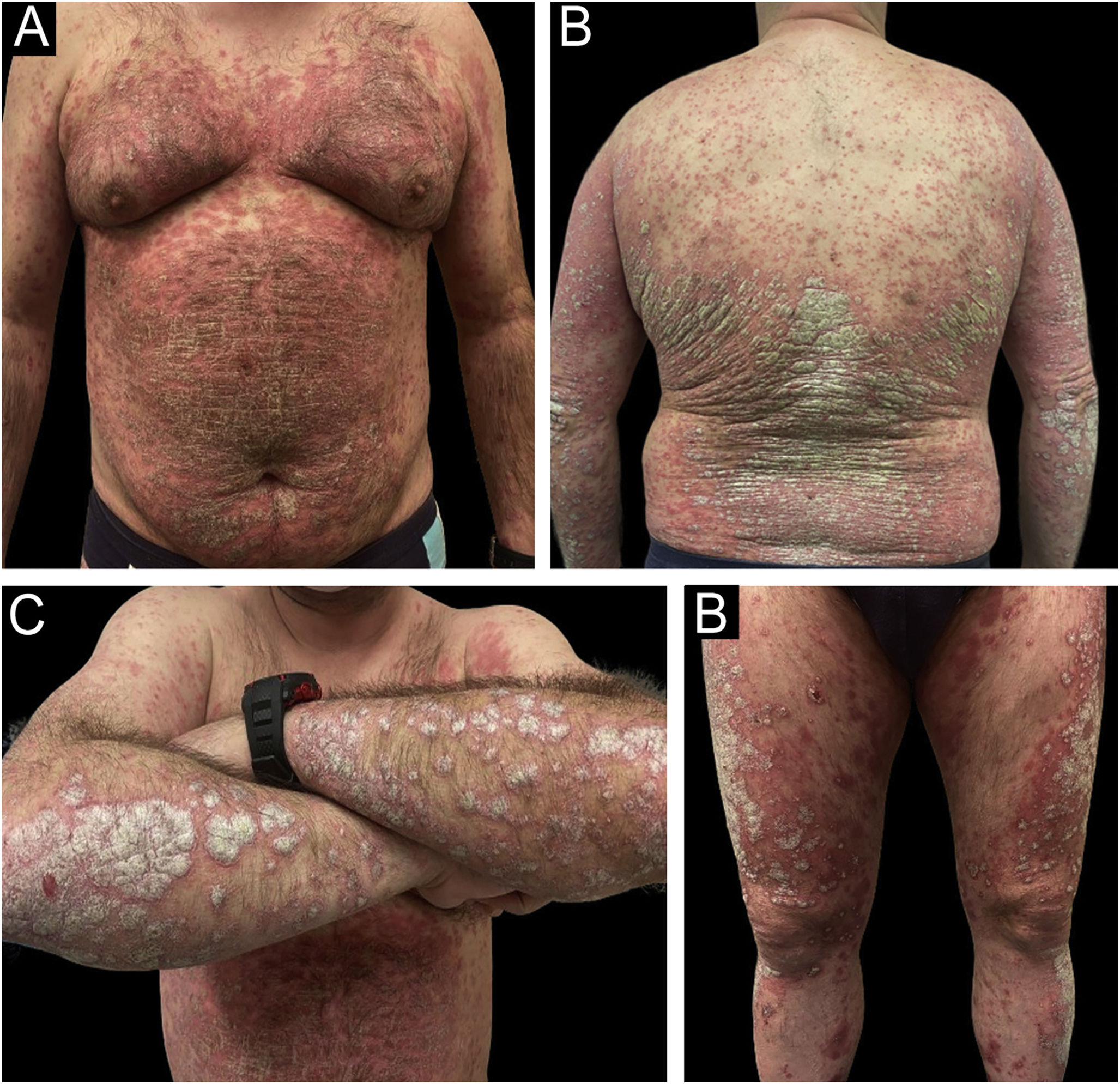
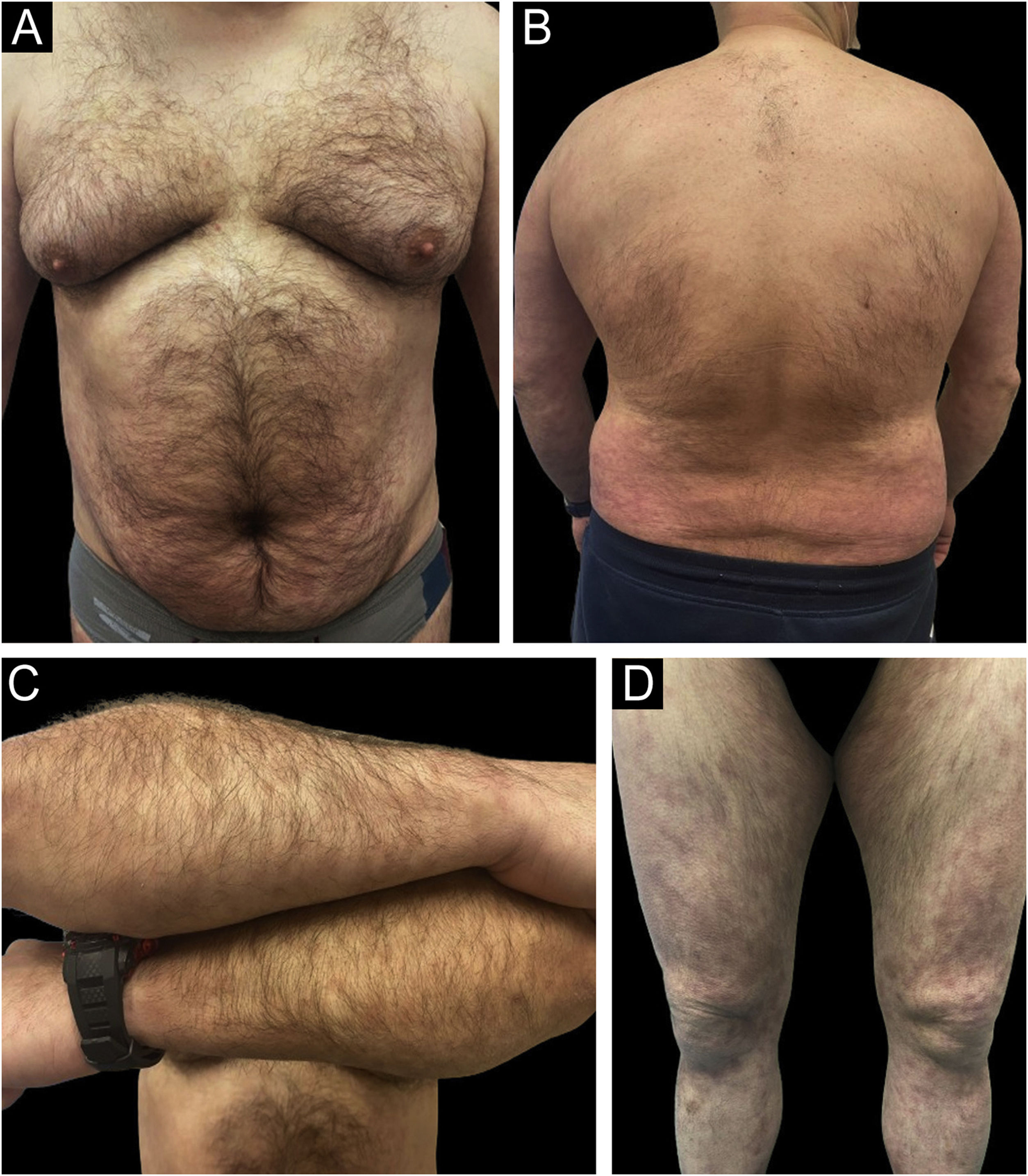
Melhoras rápidas e sustentadas ao longo do tempo foram observadas nas variáveis estudadas com todos os três medicamentos (PASI, ASC, DLQI, IGA, resposta ungueal, no couro cabeludo, área genital e envolvimento de pregas cutâneas), com excelente perfil de segurança (exemplo de paciente tratado com risanquizumabe pode ser visto nas figs. 2 e 3). Para a realização do estudo estatístico da figura 1, foram realizados testes de normalidade para todos os dados por meio do teste de Shapiro‐Wilk, detectando‐se a não normalidade. Portanto, para avaliar as quatro variáveis ao longo do tempo (valor basal – valor da semana 48‐52) em relação a cada um dos três medicamentos atuais, foi empregado o teste não paramétrico de Kruskal‐Wallis. Apenas dois efeitos adversos (náuseas e astenia) foram registrados em dois pacientes diferentes, ambos discretos e não claramente relacionados ao tratamento. Na comparação direta, não foram encontradas diferenças estatisticamente significantes entre os três medicamentos em relação a nenhum parâmetro, nem em termos de eficácia nem de segurança.
A IL‐23 desempenha papel crucial na ativação dos linfócitos T e na produção de outras citocinas pró‐inflamatórias, como a IL‐17. Ao inibir a IL‐23, os medicamentos anti‐IL‐23 reduzem a resposta inflamatória subjacente na psoríase, diminuindo assim a proliferação de queratinócitos e a formação de placas cutâneas características da doença.3 A eficácia e segurança dos três medicamentos anti‐IL‐23 aprovados (risanquizumabe, tildraquizumabe, guselcumabe) foram demonstradas em ensaios clínicos e na prática clínica,4–6 e os resultados do presente estudo apoiam esse fato. Dois estudos anteriores, por meio de análises de comparação indireta, sugerem que as três alternativas seriam comparáveis em termos de eficácia e segurança, embora sugiram que o tildraquizumabe possa ter eficácia ligeiramente inferior.7,8 Um estudo recente (que seja de conhecimento dos autores, o único até o momento) comparando os diferentes medicamentos anti‐IL‐23 na psoríase argumenta que todos os três apresentam eficácia e segurança semelhantes, sem diferenças entre eles.9 Os resultados do presente estudo são semelhantes e também não foram encontradas diferenças entre os três grupos, embora o pequeno tamanho da amostra limite o poder estatístico dessa comparação e a possibilidade de análise de subgrupos (gravidade da psoríase, localização, artrite psoriásica etc.).
As principais limitações do presente estudo são o pequeno tamanho amostral, sua natureza retrospectiva e o seguimento limitado do tratamento de um ano.
Em conclusão, guselcumabe, tildraquizumabe e risanquizumabe mostraram redução ao longo do tempo nas medidas biológicas (PASI, DLQI, ASC, IGA, resposta do acometimento do couro cabeludo, ungueal, genital, de dobras cutâneas) para o tratamento da psoríase. Não foram encontradas diferenças significantes na evolução do efeito ao longo do tempo entre os diferentes tratamentos – todos os três tratamentos funcionaram igualmente. Estudos com amostras maiores são necessários para corroborar esses resultados e investigar a presença de diferenças entre os três inibidores de IL‐23 atualmente utilizados no tratamento da psoríase.
Suporte financeiroNenhum.
Contribuição dos autoresMiguel Mansilla‐Polo: Manejo de tratamentos e procedimentos clínicos, contribuindo para o desenvolvimento desse artigo, teve acesso aos dados e desempenhou um papel na redação desse manuscrito.
Guillermo Bargues‐Navarro: Manejo de tratamentos e procedimentos clínicos, contribuindo para o desenvolvimento desse artigo, teve acesso aos dados e desempenhou um papel na redação desse manuscrito.
Conrad Pujol‐Marco: Manejo de tratamentos e procedimentos clínicos, contribuindo para o desenvolvimento desse artigo, teve acesso aos dados e desempenhou um papel na redação desse manuscrito.
Rafael Botella‐Estrada: Supervisionou o trabalho, teve acesso aos dados e participou da redação desse manuscrito.
Antonio Sahuquillo‐Torrlaba: Supervisionou o trabalho, teve acesso aos dados e desempenhou um papel na redação desse manuscrito.
Conflito de interessesNenhum.
Como citar este artigo: Mansilla‐Polo M, Sahuquillo‐Torralba A, Pujol‐Marco C, Bargues‐Navarro G, Botella‐Estrada R. Guselkumab, Risankizumab, and Tildrakizumab demonstrate parallel effectiveness and safety in psoriasis treatment: a head‐to‐head comparative study in real clinical practice. An Bras Dermatol. 2024;99:922–7.
Trabalho realizado no Hospital Universitario y Politécnico La Fe, Valência, Espanha.