Background Most of the organism’s vitamin D (VD) is obtained through the cutaneous synthesis after exposure to the sun’s UVB radiation. Sunscreens are indicated for the prevention of actinic damage to the skin, however, there are few clinical trials assessing the synthesis of cutaneous VD in real-life situations of sun exposure with ordinary clothing and usual photoprotection.
Objectives To evaluate the synthesis of VD with suberythemal sun exposure in healthy adults using topical photoprotection (SPF 30).
Methods: Quasi-experimental study, conducted at Rio de Janeiro (Brazil), during winter, with 95 healthy adults who had 25-OH-VD checked twice, 24 hours apart, and were exposed to the sun (UVB=20 mJ/cm2), according to a randomized grouping: SC - use of SPF 30 on the face, neck and chest (n=64), NO - no sunscreens (n=10), CO - confined from sun exposure for 24h (n=21). The groups were matched according to the propensity score related to gender, age, phototype, body mass index, glycosylated hemoglobin and baseline levels of VD. The outcome evaluated was the variation (ΔVD) in serum level of 25-OH-VD (ng/ml) between the groups.
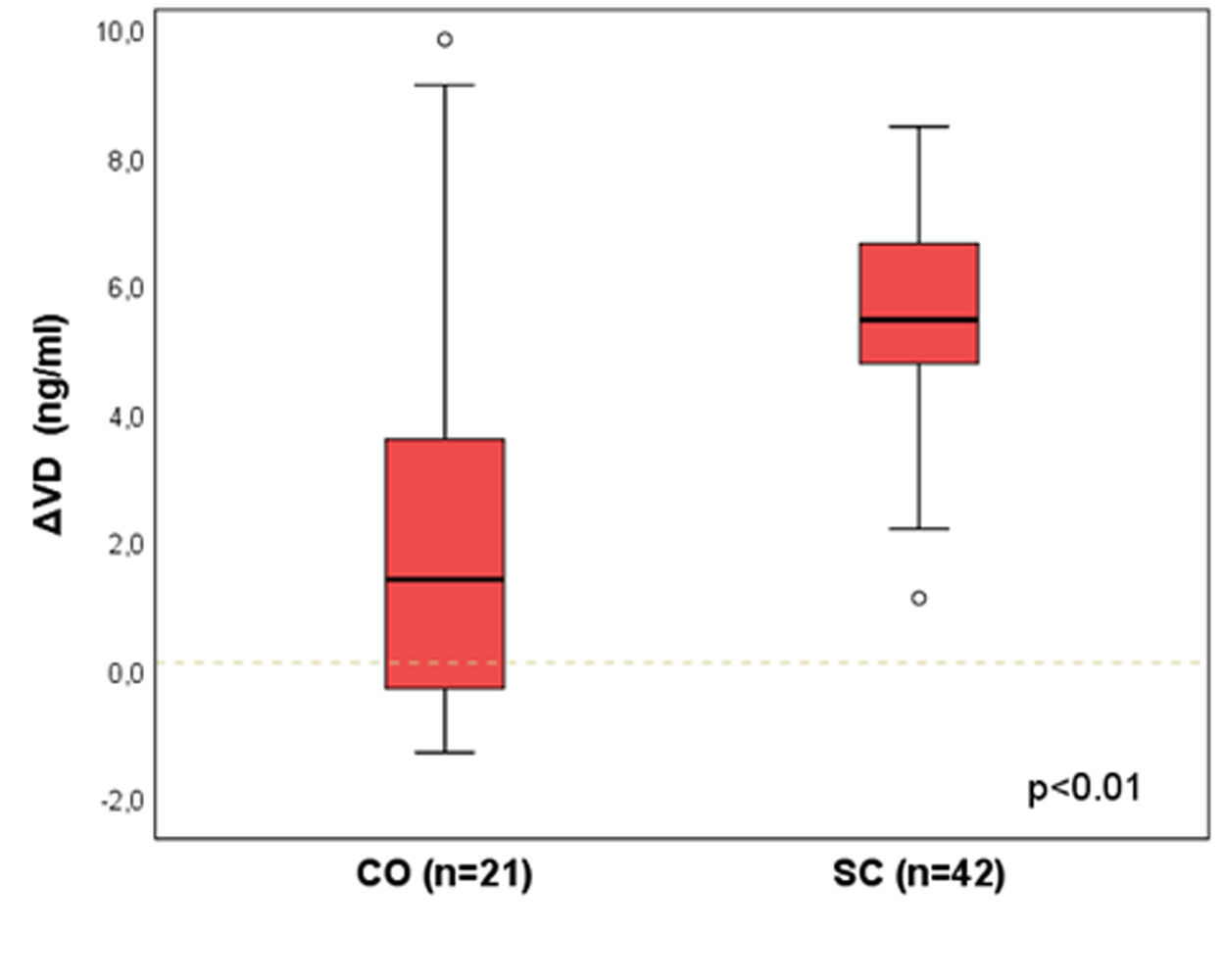
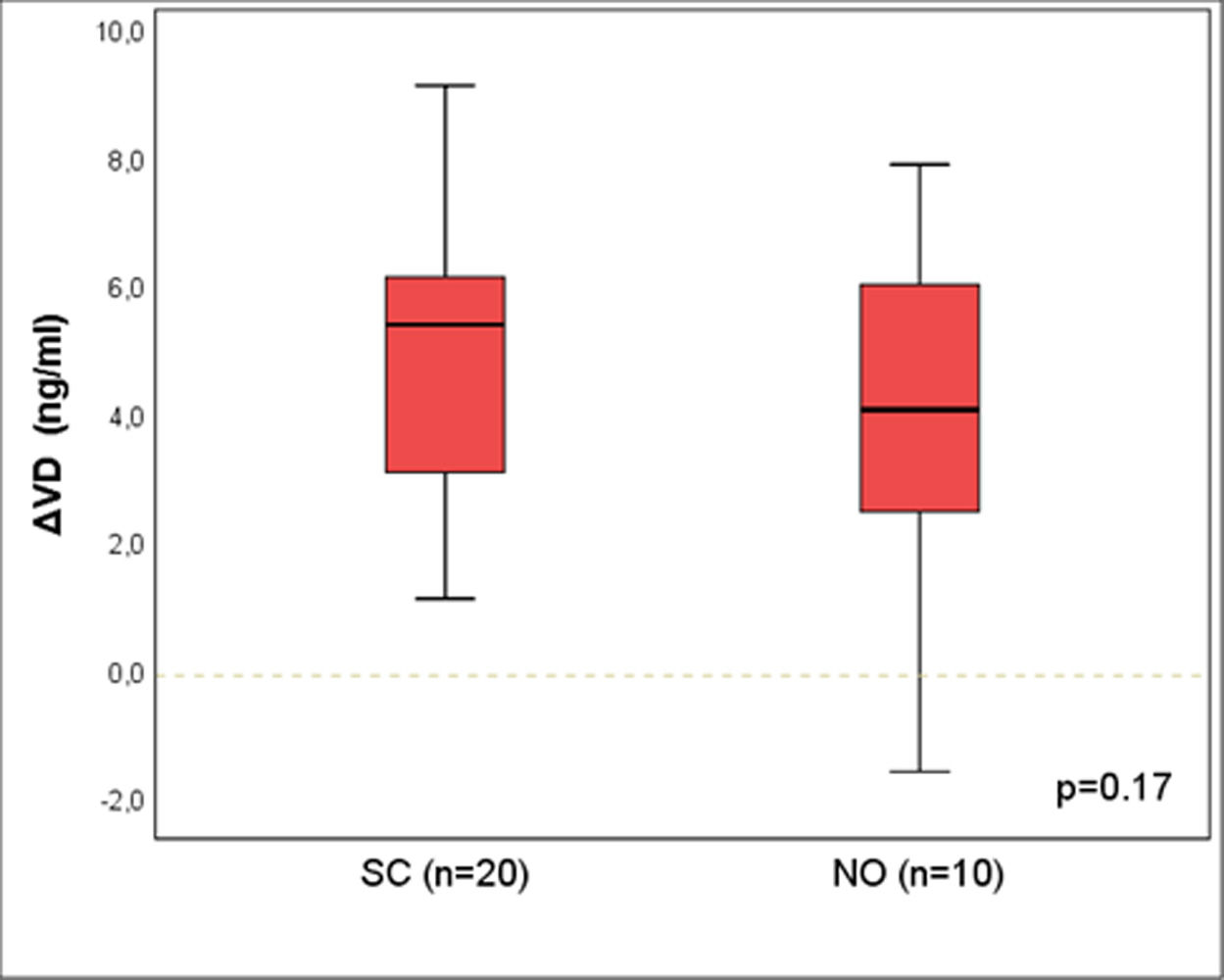
Results: A statistically significant difference was identified between CO and SC groups [median (p25-p75)]: ΔVD =1.4 (-0.3-3.6) vs. 5.5 (4.8-6.6); p<0.01. There was no difference between SC and NO groups: 5.4 (3.1-6.1) vs. 4.1 (2.5-6.0); p=0.17.
Study limitations:Laboratory analysis technique (chemiluminescence) with great variability, loss of food intake standardatization, unbalanced groups.
Conclusions: Suberythemal sun exposure with sunscreen (SPF 30) provides similar vitamin D serum variation than without photoprotection in healthy adults.
Vitamin D (VD) is a steroid hormone that acts in a genomic and non-genomic way, in different metabolic processes in most tissues. Its deficiency leads to osteomuscular damage (for example, rickets, decreased physical strength, osteoporosis) and there is indication that it favors infertility, immune and cardiovascular disturbances, development of autoimmune diseases and some malignancies.1-5
In the last decades, there was an increase in the diagnosis of hypovitaminosis D all over the world.6 A study conducted in São Paulo (Brazil) with 603 healthy volunteers, identified 77% as having insufficient and 19% deficient VD. The factors associated to these findings were: advanced age, darker skin and higher body weight; such numbers are comparable to studies performed in countries with lower temperatures and solar radiation than Brazil. 7,8
Synthesis of the active form of VD (1.25-OH-VD) is regulated by the calcium, phosphorus, parathyroid hormone, fibroblast growth factor, medication levels, besides the activity of liver and kidney hydroxylases. The two substrate sources for the synthesis of 1.25-OH-VD are diet and photolysis of 7-dehydrocholesterol in the keratinocyte membrane by ultraviolet radiation B (UVB=290-315 nm), according to the temperature of the skin. Usually, more than 90% of VD is derived from cutaneous photosynthesis with suberythemal sun exposure, since the diet alone hardly provides the daily recommended doses, especially for at-risk populations.9-11
Considering that sunscreens effectively block UVB, some experts advocate that their regular use can lead to hypovitaminosis D. The main study that subsidizes this hypothesis was performed with narrowband UVB (nbUVB) phototherapy devices, where 8 nude participants received minimal erythema doses (MED) with sunscreen all over the skin; that resulted in a marked difference in the synthesis of VD between the protected and the unprotected group.12
Sunscreens are indicated for the prevention of actinic damage to the skin, however, there are few studies investigating the cutaneous synthesis of VD in real-life situations of sun exposure, with ordinary clothing and usual topical photoprotection.
The objective of this study was to evaluate the synthesis of VD triggered by suberythemal sun exposure in healthy adults using topical photoprotection (SPF 30), through the variation of plasma levels of 25-OH-VD, within 24h. In addition, the factors associated to the synthesis of vitamin D were explored.
MethodsAn open, parallel, quasi-experimental study was conducted in the city of Rio de Janeiro (Brazil), latitude -23.00oS, on the 4th and 5th of August 2017, during the II Simpósio Internacional de Cabelos e Unhas. 13
The project was approved by the Committee of Ethics in Research of the UFF (CAAEE n. 69912217.0.0000.5243), and all participants signed a consent form.
Population of the studyWere included in the study: adults (20-70 years) of both genders and Fitzpatrick phototypes I to V who agreed to have VD dosed twice, 24 hours apart, and expose to the sun for a specified time according to the randomization group.
The groups that exposed to the sun with photoprotector (SC) or without photoprotector (NO) were made up of dermatologists, medical students, residents and other participants of the event.
The group termed as confined (CO) was arranged for convenience among dermatologists, professors, medical students and residents willing not to expose to the sun for 24h.
These individuals were not included in the study: cirrhotic, with renal disease, taking supplementary VD the week prior, albinos or those with allergy to sunscreens.
The participants that did not complete one of the dosages of VD or that deviated from the protocol of sun exposure were excluded from the study (analysis per protocol).14
InterventionAfter the inclusions, all participants were randomized (8:2) into the SC and NO groups. The distribution was according to the protocol of Zelen.15
All participants answered a clinical and demographic questionnaire and were submitted to fasting peripheral blood collection for the baseline dosage of VD and glycosylated hemoglobin, between 7 and 9 am on August 4th, 2017 and proceed to attend the event, protected from the sun. At noon, they underwent a suberythemal sun exposure (20mJ/cm2), with the clothing used on the day of the event.
The participants in the SC group applied sunscreen liberally (Anthelios Airlicium 30, La Roche Posay) on the face and usual areas before sun exposure.
UVB dosing was conducted using the device Digital UVB Radiometer (Zoo Med Laboratories, Inc; USA) and endorsed by the local ultraviolet index (https://www.climatempo.com.br/uv/321/riodejaneiro-rj).
In the morning of August 5th there was a second fasting blood collection, since the peak of VD photosynthesis takes 24h to occur.16
The tubes of both samples were immediately stored in Styrofoam with ice and sent away to the laboratory at HUAP-UFF. All samples were processed in the same laboratory, using the chemiluminescence technique (ARCHITECT 25-OH Vitamin D, Abbott Diagnostics, Lake Forest, IL, USA).
There was no dietary or clothing restriction during the 24h of the experiment, only regarding sun exposure.
Statistical analysisThe main outcome evaluated was the variation in the serum levels of 25-OH-VD (ng/ml), defined by the difference between baseline and 24h levels (ΔVD). The sun exposure regimens: confined (CO), sunscreen use (SC) or no photoprotection (NO), were the main independent variables. The study’s co-variables were the baseline levels of 25-OH-VD, gender, age, Fitzpatrick phototype (I-V), body mass index (BMI – kg/m2) and glycosylated hemoglobin (%).
The data were analyzed according to the population per protocol, i.e., that were correctly distributed in the pre-defined groups and had two blood samples. 14
Quantitative variables were represented by means and standard deviations or medians and quartiles (p25-p75), if the normality parameters were not confirmed by the Shapiro-Wilk test.17 Qualitative variables or ordinals were represented by percentages.
The bivariate comparison of the co-variables between the groups was performed using Pearson’s Chi-square test (quantitative data), Chi-square for trend (ordinal data) and Kruskal-Wallis (qualitative data).18
Matching of the SC and CO and NO groups was in the proportion of 2:1, from a propensity score (next-neighbor, without reposition) of the described co-variables.19
The comparison between the values of ΔVD between the groups was performed by a generalized linear mixed effects model (gamma distribution of probability, identity function, unstructured matrix, robust covariance matrix and variable intercept).20
Posteriorly, the analysis of the sensitivity of the results was performed including all participants of the groups SC, CO and NO, via a generalized linear model (gamma distribution of probability, identity function, robust covariance matrix), adjusted by the co-variables.21 The post-hoc correction for multiple tests was performed by the method of Bonferroni.22
The ΔVD values of the SC group were evaluated according to the co-variables by a generalized linear model (normal probability distribution, identity function, robust covariance matrix).18 The diagnosis of the models was based on the AIC criterion, the evaluation of the normality of the residuals and the linearity between the expected and observed values.
The data were analyzed with the software IBM SPSS 25.
We considered significant a value of p<0.05.
Sample sizeThe study of the difference of VD synthesis according to photoprotection was sized for the detection of a variation (ΔVD) of more than 2ng/ml, with the same standard deviation of the differences (2ng/ml) for matched, unbalanced groups (2:1), with 90% power and two-tailed alpha level of 0.05 (comparison SCxCO); resulting in a total of 14 participants in the CO group. For a one-tailed comparison with the same parameters, it results in 10 participants in the NO group.23
The second study (factors associated to the synthesis of VD) was sized to meet an exploratory generalized linear model with up to six co-variables, according to the Freeman formula, resulting in a minimal sample of 60 participants (group SC).24
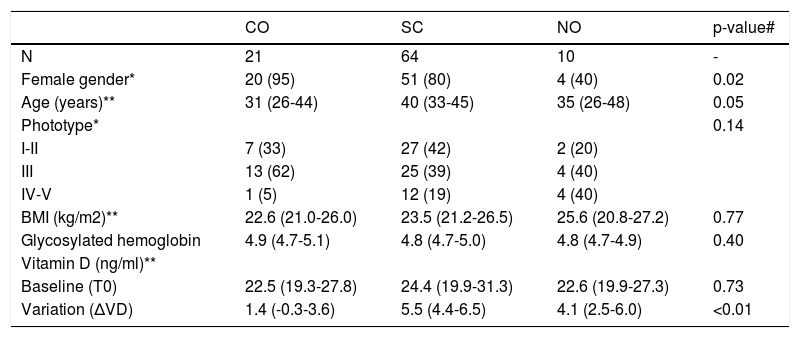
ResultsOf the 113 participants selected, 95 performed the second measurement of VD and formed the population per protocol. The main clinical data and those related to VD are shown in table 1. Baseline VD levels below 30ng/ml (insufficiency) were identified in 70 (74%) participants, with 26 (27%) presenting with levels below 20 ng/mg (deficiency).11
Main clinical and laboratory data of the groups sampled
| CO | SC | NO | p-value# | |
|---|---|---|---|---|
| N | 21 | 64 | 10 | - |
| Female gender* | 20 (95) | 51 (80) | 4 (40) | 0.02 |
| Age (years)** | 31 (26-44) | 40 (33-45) | 35 (26-48) | 0.05 |
| Phototype* | 0.14 | |||
| I-II | 7 (33) | 27 (42) | 2 (20) | |
| III | 13 (62) | 25 (39) | 4 (40) | |
| IV-V | 1 (5) | 12 (19) | 4 (40) | |
| BMI (kg/m2)** | 22.6 (21.0-26.0) | 23.5 (21.2-26.5) | 25.6 (20.8-27.2) | 0.77 |
| Glycosylated hemoglobin | 4.9 (4.7-5.1) | 4.8 (4.7-5.0) | 4.8 (4.7-4.9) | 0.40 |
| Vitamin D (ng/ml)** | ||||
| Baseline (T0) | 22.5 (19.3-27.8) | 24.4 (19.9-31.3) | 22.6 (19.9-27.3) | 0.73 |
| Variation (ΔVD) | 1.4 (-0.3-3.6) | 5.5 (4.4-6.5) | 4.1 (2.5-6.0) | <0.01 |
The estimated ultraviolet index during the exposure was 5, temperature was 23oC and dosage of UVB ranged between 70 to 170 mW/cm2. That led to an adjusted sun exposure for each individual between 14 and 18 minutes in order to achieve a minimum of 20mJ/cm2.
Figure 1 presents the ΔVD results between the groups CO and SC, matched by propensity score (n=21 and 42). A statistically significant difference was identified between the groups [median (p25-p75)]: ΔVD = 1.4 (-0.3-3.6) vs. 5.5 (4.8-6.6); p<0.01.
Figure 2 shows the results for ΔVD between the groups SC and NO, matched by propensity score (n=20 and 10), and no difference was seen between the groups [median (p25-p75)]: ΔVD = 5,4 (3,1-6,1) vs. 4,1 (2,5-6,0); p=0,17.
Sensitivity analysis performed with all participants (n=95), comparing the ΔVD between groups, adjusted for all co-variables, identified a significant difference between SC and CO (p<0.01) and no difference between SC and NO (p=0.68), confirming the results with matched participants.
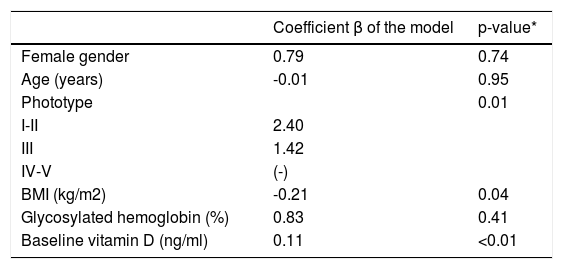
The ΔVD values among the 64 participants in group SC were positively associated to lighter phototypes and higher baselines levels of VD; however, negatively to BMI (Table 2).
Variation in the values of vitamin D, according to the main co-variables in the study (n=64)
| Coefficient β of the model | p-value* | |
|---|---|---|
| Female gender | 0.79 | 0.74 |
| Age (years) | -0.01 | 0.95 |
| Phototype | 0.01 | |
| I-II | 2.40 | |
| III | 1.42 | |
| IV-V | (-) | |
| BMI (kg/m2) | -0.21 | 0.04 |
| Glycosylated hemoglobin (%) | 0.83 | 0.41 |
| Baseline vitamin D (ng/ml) | 0.11 | <0.01 |
p(model)<0.01; p(constant)<0.01; AIC = 294
The results identified variation in plasma levels of VD with the use of photoprotection in adults mildly exposed to the sun, in levels that did not differ from the participants that exposed without sunscreen. Since the synthesis and metabolism of VD depend on multiple factors, real-life clinical trials are important to substantiate recommendations for clinical practice.25
Maia et al selected 50 adults, matched by gender and age, Fitzpatrick phototype III in winter in São Paulo (Brazil). Half was using photoprotection as advised by a dermatologist and were compared to their controls, who exposed to the sun without photoprotection. Even though the levels of VD were lower among the photoprotected (medians: 23.1 vs. 35.4 ng/ml), no cases deficient in VD (<20 ng/ml) or with abnormal parathyroid hormone were identified in either group.26 The authors concluded that usual sun exposure ensures an adequate synthesis of VD.
Individuals with a history of skin cancer or with other photodermatoses also adopt sun exposure-avoiding behaviors besides topical photoprotection, what can represent a confounding element in observational studies.27 This happened in the study by Matsuoka et al, that compared 20 adults with skin cancer history who, in the course of 12 months, applied sunscreen on the exposed parts of the body to controls living in the same area.28 The authors identified lower levels of VD among the patients that used sunscreen and concluded that the chronic use of sunscreen would lead to low levels of VD, however, they did not conducted baseline testing prior to the intervention.
Hansen et al conducted a study in Denmark with 3194 participants, where the use of sunscreen was not associated to low levels of VD in children or adults. On the contrary, these individuals had higher chances of levels > 50nmol/L, possibly by the practice of more intense sun exposure among sunscreen users. On the other hand, practices such as shade-seeking and the use of protective clothing were correlated to lower levels of VD.29
Libon et al evaluated 72 volunteers submitted to a single 0.8 MED irradiation (nbUVB) using 2mg/cm2 photoprotection (FPS 50+) and clothing that allowed for the exposure of different body parts, incurring in a relative reduction of 8-13% in the serum levels of VD.30 The authors concluded that the use of sunscreen has little impact in serum levels of VD.
The chronic use of photoprotection was investigated by Marks et al in 113 adults (>40 years) with actinic lesions, randomized to use sunscreen (SPF 17) or placebo, during the Australian summer.31 There was an increase in 25-OH-VD with no difference between groups and, as in our study, no effect of age or gender was observed. None of the participants had below normal levels of VD.
Another Australian study by Kimlin et al evaluated factors associated to VD levels in 126 individuals between 18 and 87 years and did not find an association between the use of sunscreen and levels of 25-OH-VD.32
Indeed, the maximum plateau of the cutaneous synthesis of VD takes place in low UVB doses regimens (1/3 of the minimum erythema dose, or about 20-30mJ/cm2), and doses nearing erythema promote the local degradation of the VD generated in the skin, in an auto-regulatory mechanism.33,34 These elements suggest that real-life customary exposure (for example, walking to work, school, lunch time, gardening, outdoors sports), such as what we tried to recreate in this study, are more effective for the synthesis of VD.
There is some UVB penetration through clothing, scalp and areas not completely covered by the sunscreen. 35 Moreover, the thickness of sunscreen application employed by most of the population does not reach the recommended 2mg/cm2, what leads to a variation in the amount of UVB that reaches the skin according to the thickness of photoprotection coverage and allows for some synthesis of VD.36,37
Besides, lighter-skinned individuals who are at higher risk for actinic lesions, are also the most effective synthesizers of VD, what minimizes the impact of sunscreen in VD synthesis. 38,39
An important part of the hypovitaminosis D epidemics that affects the world population can originate from inadequate diet and, mainly from leisure activities (for example, shopping malls, gymnasiums, air-conditioned environments) protected from direct sun exposure of protected by glass (at home or in the car), that are features of the lifestyle of modern urban civilization, instead of being caused only by photoprotection measures.40 Polymorphisms in the receptor of VD are also implicated in clinical effects, even when the serum levels are appropriate.41
The Brazilian Consensus on Photoprotection recommends that intentional and unprotected sun exposure should not be indicated as a source for the production of VD. Besides, patients at risk for developing hypovitaminosis D should receive oral supplementation.42
Largest variations in the levels of VD were identified among lighter phototypes of the participants in the group SC. In fact, the most accepted hypothesis for the differentiation of skin color tones in the planet is due to the evolutional gain from VD photosynthesis and to the photolysis of folic acid in the skin by UVA.43,44 People that migrated from Africa to higher latitudes, with a lower incidence of UVB, such as subtropical areas of Europe, were selected to have lighter skin due to the high demand of VD during pregnancy, lactation and child development. In parallel, in intensely sunny regions, the melanization of the skin was maintained to avoid folic acid depletion, essential for the development of the neural tube, fertility and hematopoiesis.44,45 Thus, there is a strong correlation between the constitutional pigmentation and the geographical distribution of the primitive people.46
Participants with higher BMI presented lower variations in the ΔVD. Since part of the 25-OH-VD produced is stored in the fat tissue, obesity promotes a higher diffusion (and lower bioavailability) of this hormone, regardless of cutaneous synthesis or oral supplementation.47
Our results did not identify an association between the variation in VD levels and age of the participants. In a study with 6 youngsters (20-30 years) and 6 elderly (62-80 years) who had their whole body irradiated with a single dose of 32mJ/cm2, youngsters had a three-times higher mean increase compared to the elderly.48 The age range of our participants was from 22 to 68 years, eventually, restricted to receive such effect in low UVB doses, however, when separately analyzed, the production of VD in those younger than 30 years did not differ from those older than 50 years (data not shown).
All participants had normal levels of glycosylated hemoglobin with low variability, what impaired the analysis of this variable for the adjustment of the values of ΔVD in relation to diabetes or insulin resistance, factors known to interfere in the metabolism of VD.49
The participants with higher baseline levels of VD were the ones that had higher variation in the levels after sun exposure. These results are contrary to what was expected, which was a higher synthesis and bioavailability of 25-OH-VD in those deficient. The same results were confirmed when the deficient (<20ng/dl) were compared to the sufficient (data not shown). Possibly, the effect of the metabolism of VD for the perception of this phenomenon takes longer than 24h and is not only controlled by the cutaneous synthesis, as seen in this investigation.9,25
This study presents potential limitations associated to the technique of laboratory analysis of VD (chemiluminescence), subject to a high variability of the measurements, even when analyzed in the same laboratory and with the same kit.50 That, however, did not preclude the differential identification of the synthesis of VD between the groups.
The unbalanced randomization due to the Zelen-type distribution (negative of unprotected sun exposure by some participants) was compensated by matching propensity score, which included all covariables of the study, as skin phototypes and body mass index. Besides, the sensitivity analysis, adjusted by the co-variables, confirmed the results of the matched groups.
The lack of standardization on food intake has low impact in VC serum levels, since normally only 10% of those levels are provided from diet.11 Likewise, the modest sample from each subgroup did not hinder the significant differences between the exposure regimens from being identified.
The generalization of the results for other populations, with different diets, clothing coverage of the body and different solar UVB irradiations should be pondered with caution.
The results of this study should be complemented by subsequent experiments, involving participants submitted to the same confinement regimens and exposed to increasing UVB doses from sun exposure in different moments, in order to verify the consistency of these findings under different conditions controlled by the same participants. The importance of the SPF in the synthesis of VD in usual conditions should also be explored. Moreover, the use of liquid chromatography for the estimation of VD could improve the accuracy of the results.11
ConclusionSuberythemal sun exposure with sunscreen (SPF 30) applied in the usual fashion, allows for the variation of plasma levels of vitamin D similar to that achieved without photoprotection in healthy adults.
Financial support: Sociedade Brasileira de Dermatologia.
Conflict of interest: None.