Mycosis fungoides (MF) is the most common subtype of cutaneous T-cell lymphoma. TNMB system is the staging method used in MF, and it not only guides therapeutic management, but represents the main prognostic factor. In order to improve the prognostic evaluation, the Cutaneous Lymphoma International Prognostic Index (CLIPi) was proposed.
Objective:To evaluate the performance of CLIPi score for prognostic analysis in patients with early stage MF.
Methods:This is a retrospective cross-sectional observational study, with exploratory analysis. The outcome variables were disease progression and related death.
Results:One hundred and two patients were stratified according to CLIPi score, being the majority classified as low risk. Patients with intermediate or high risk presented disease progression more frequently than those with low risk (PR: 1.2 / p = 0.004 / 95%CI: 1.0 – 1.6). The same did not occur with the variable related death. In addition, survival rates were not consistent with risk stratification.
Study Limitations:: Small sample and its retrospective analysis.
Conclusions:Since CLIPi score was proposed, four other studies that we could consult showed conflicting results, similar to the present study. Further studies are necessary for a recommendation of its use.
Mycosis fungoides (MF) is the most common subtype of cutaneous T-cell lymphoma (CTCL) and has a behavior classified as indolent, with an overall survival in five years of 88%.1-4
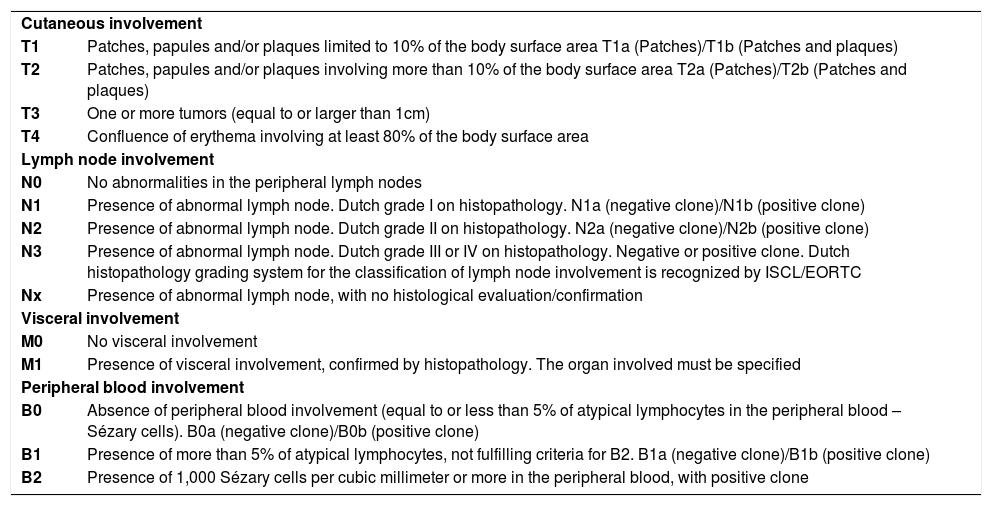
The current staging is through the TNMB system, proposed by the Mycosis Fungoides Cooperative Group (MFCG) and subsequently revised by the International Society for Cutaneous Lymphomas (ISCL) and by the European Organization of Research and Treatment of Cancer (EORTC) (Charts 1 and 2). In it, the letter T represents the cutaneous involvement and extent of the disease; N, lymph node involvement; M, the presence or absence of metastasis; and B, extension to peripheral blood.4,5TNMB staging system, albeit not ideal, remains as the main prognostic factor.5
TNMB staging of MF-type CTCL
| Cutaneous involvement | |
| T1 | Patches, papules and/or plaques limited to 10% of the body surface area T1a (Patches)/T1b (Patches and plaques) |
| T2 | Patches, papules and/or plaques involving more than 10% of the body surface area T2a (Patches)/T2b (Patches and plaques) |
| T3 | One or more tumors (equal to or larger than 1cm) |
| T4 | Confluence of erythema involving at least 80% of the body surface area |
| Lymph node involvement | |
| N0 | No abnormalities in the peripheral lymph nodes |
| N1 | Presence of abnormal lymph node. Dutch grade I on histopathology. N1a (negative clone)/N1b (positive clone) |
| N2 | Presence of abnormal lymph node. Dutch grade II on histopathology. N2a (negative clone)/N2b (positive clone) |
| N3 | Presence of abnormal lymph node. Dutch grade III or IV on histopathology. Negative or positive clone. Dutch histopathology grading system for the classification of lymph node involvement is recognized by ISCL/EORTC |
| Nx | Presence of abnormal lymph node, with no histological evaluation/confirmation |
| Visceral involvement | |
| M0 | No visceral involvement |
| M1 | Presence of visceral involvement, confirmed by histopathology. The organ involved must be specified |
| Peripheral blood involvement | |
| B0 | Absence of peripheral blood involvement (equal to or less than 5% of atypical lymphocytes in the peripheral blood – Sézary cells). B0a (negative clone)/B0b (positive clone) |
| B1 | Presence of more than 5% of atypical lymphocytes, not fulfilling criteria for B2. B1a (negative clone)/B1b (positive clone) |
| B2 | Presence of 1,000 Sézary cells per cubic millimeter or more in the peripheral blood, with positive clone |
*Adapted: Olsen E et al., 2007.5
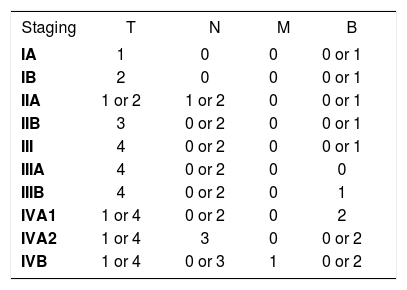
At the time of diagnosis, most patients have early stage disease, defined by the TNMB staging as stage IA (T1N0M0), IB (T2N0M0) or IIA (T1 or 2N1 or 2M0) (Charts 1 and 2). Four large published cohorts found in descending order, 78.3%, 75.4%, 71.5% and 66% of patients with stage IA-IIA at the time of diagnosis.6-9
In prognostic terms, patients diagnosed with stage 1A MF have survival rates similar to those of the general population when matched by age.1,7,9 From stages IB and IIA, there is already an impact on the survival, even though they are still conceptually classified as early stage MF. A cohort study published by Desai et al.in 2015 with 393 patients, demonstrated a 5-year survival of 86.8% and 90.3% for stages IB and IIA, respectively.6 Similar results were found in the study published by Agar et al., with 1,502 patients, where 5-year survival was 84% for IB and 78% for IIA.10 The drop in survival occurs more significantly from stage IIB, when MF are no longer considered early stage and start to be considered as having advanced MF. In the previously mentioned cohorts, 5-year survival in stage IIB was of 28.1% and 47%, respectively.6,10
Approximately 20 to 25% of patients with stage I MF progressed to more advanced stages of the disease, with significant impairment of survival. Predicting which patients are under a higher risk of progression is still challenging, since there are no adequate prognostic markers.11
With the aim of improving the prognostic assessment of MF patients, Benton et al. suggested the implementation of a prognostic score, known as Cutaneous Lymphoma International Prognostic Index (CLIPi) (Chart 3).12 For this purpose, they studied 1,503 cases, published by Agar et al.10Validation of the score involved 1,221 outpatients. The score is differentiated for early stage and late stage MF an utilizes independent prognostic factors, with more statistical importance. They are: male gender and age, evaluated in both stages of MF; plaque lesions, folliculotropism, and N1 lymph node involvement (TNMB) for early stage MF, and B1/B2, N2/N3 visceral involvement for late stage MF. According to the presence of these characteristics, all scoring the same value (1 point), the patient is stratified into: low risk (0 to 1, i.e., none or at least 1 factor present), intermediate risk (2, i.e., 2 factors present) and high risk (3 to 5, i.e., between 3 and all 5 factors present). We highlight that the combination of the factors does not alter the proposed stratification, i.e., when 2 points are reached, either by being male and having plaque lesions, or by age and folliculotropism, it is still considered intermediate risk and so forth.12
CLIPi score
| Early stage MF: |
| Male genderAge > 60 yearsPlaquesFolliculotropismN1/NX (TNMB staging)Interpretation: 0 – 1 (low risk)/2 (intermediate risk)/3 – 5 (high risk) |
| Late stage MF: |
| Male genderAge > 60 yearsB1/B2 (TNMB staging)N1/NX (TNMB staging)Visceral involvement |
| Interpretation: 0 – 1 (low risk)/2 (intermediate risk)/3 – 5 (high risk) Adapted: Benton EC et al., 2013.12 |
The objective of this study was to evaluate the performance of CLIPi score as a prognostic factor in a sample of early stage MF patients (stages IA and IB only), treated at the Sector of Photodermatology, Dermatology Division, Hospital Universitário Clementino Fraga Filho – Universidade Federal do Rio de Janeiro (HUCFF/UFRJ), and compare our data to the other studies published.
MethodsThis is an observational, cross-sectional, retrospective, exploratory data analysis study. The population of the study comprehended patients seen at the Sector of Photodermatology at HUCFF/UFRJ, between January 2000 and December 2015, who were diagnosed with MF.
It was considered a case of MF the patient who had the three following criteria:
· Patches (only changes in the color of the skin, with no relief or texture changes) or plaques (raised, flat lesion, larger than 1cm in diameter), that were hypopigmented or erythematous-coppery, with or without scaling, of different sizes and preferably affecting photoprotected areas;13
· Chronic course (at least 6 months), persistent or progressive;
· Consistent histopathology (taking into consideration the clinical-pathological correlation). The presence of a lymphoid infiltrate along the dermal-epidermal junction, accompanied by epidermal invasion of lymphocytes without spongiosis or lymphocyte atypia (large, irregular, hyperchromatic nuclei) can be included in this situation.
Were excluded from the analysis patients with:
· Staging IIA or higher (i.e., disease not restricted to the skin);
· Insufficient data in the patients file;
· Positive serology for HTLV 1/2;
· Diagnosis of other associated lymphomas;
· Follow-up of less than 5 years.
The dependent variables analyzed were:
· Progression of the disease as staging, classified as qualitative, dichotomous and nominal. Since stage IA and IB patients were included in the study, it was considered disease progression those who evolved to stage IIA onwards.
· Death related to the disease (being the lymphoma itself the cause of death, or deaths related to complications of systemic therapies used). The variable disease-related death was also treated in a qualitative, dichotomous and nominal fashion.
The independent variables studied were established by Benton et al.for the development of a specific early stage MF CLIPi score(Chart 3)12:
· Male gender;
· Age over 60 years;
· Plaque lesions;
· Folliculotropism.
All independent variables were treated in a qualitative, dichotomous and nominal fashion. Lymph node spread was not considered, since only TNMB IA or IB patients were included (Charts 1 and 2).
According to the number of independent variables present, the patients were classified in low, intermediate and high risk, as per the interpretation proposed by the authors of the article who support the prognostic score. Subsequently, low-risk patients were differentiated from those of intermediate and high risk, allowing for the creation of double entry tables and statistical analysis.
The data selected were grouped into printed spreadsheets and digitalized with Excel 2011 (Microsoft® Excel® for Mac 2011/Version: 14.2.0). Data were analyzed with the aid of the statistical software SPSS, version 24.0. the studies of the association between categorical data and dependent variables were performed using chi-squared test or Fischer’s exact test. As measures of association, prevalence ratios and their respective confidence intervals (CI: 95%) were calculated. The criterion of significance used was the 5% level.
The study is in accordance with the resolution 466/12 of the National Council of Health. It is registered at Plataforma Brasil and was approved by the Ethics Committee at HUCFF/UFRJ (CAAE 59235916.9.0000.5257). The researches were responsible for the privacy and confidentiality of the data collected, fully preserving patient anonymity.
ResultsOne hundred and two patients were included out of a total of 135 patients selected from a patient registry seen at the Sector of Photodermatology.
Among the records of 33 excluded patients, 17 had incomplete data or insufficient follow-up; 10 had other diagnoses (parapsoriasis, lymphomatoid papulosis, cutis laxa) and 6 had positive serology for HTLV 1/2.
Among the 102 patients studied, 30 (29.4%) presented disease progression during follow-up and 8 died (7.8%) from the disease or from treatment-related complications.
Only the presence of plaque lesions had a significant p-value regarding the association with stage progression in a bivariate analysis. However, confidence interval values included 1.0. This association was not maintained regarding the occurrence of MF-related deaths (Table 1).
Analysis of the frequency of independent variables and calculation of the prevalence ratio with the association to dependent variables
| Variables independent | Dependent variables | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progression | Related death | |||||||||||
| Nº | Freq (%) | Nº | Freq (%) | P R | P | CI 95% | Nº | Freq (%) | PR | P | 95% CI | |
| Male | 55 | 53.9 | 19 | 34.5 | 1.4 | 0.1 | 0.7 – 2.7 | 4 | 7.3 | 0.8 | 0.5 | 0.2 – 3.2 |
| > 60 years | 50 | 49.0 | 16 | 32.0 | 1.1 | 0.3 | 0.6 – 2.1 | 3 | 6.0 | 0.6 | 0.3 | 0.1 – 2.4 |
| Plaques | 56 | 54.9 | 22 | 39.3 | 1.3 | 0.01 | 1.061 – 1.745 | 6 | 10.7 | 1.0 | 0.2 | 0.9 – 1.1 |
| Folliculotropism | 6 | 5.9 | 1 | 16.7 | 0.5 | 0.4 | 0.1 – 3.3 | 0 | 0 | - | - | - |
| N1/Nx | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
N: number. Freq: frequency, expressed in percentage. PR: prevalence ratio. 95% CI: 95% confidence interval
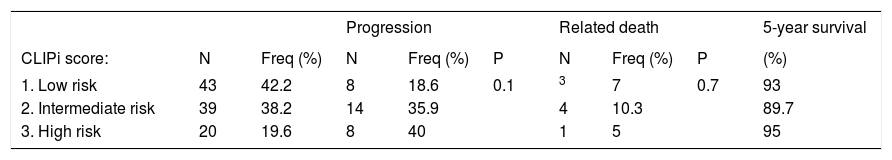
As for the distribution of CLIPI score according to disease progression and disease-related deaths, the results are shown in table 2. Patients with intermediate and high risk evolved to disease progression in 35.9% and 40% of the cases, respectively. Lower rates of survival were seen in intermediate risk patients (89.7%). The analysis in double entry table, differentiating the risks into associated low and intermediate and high risk and their correlation with the outcome variables is shown in table 3. The combined group of intermediate- and high-risk patients had a prevalence ratio of 1.4 (p=0.04).
:Distribution of the frequency of classification of the CLIPi score according to the frequencies of progression and related death
| Progression | Related death | 5-year survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CLIPi score: | N | Freq (%) | N | Freq (%) | P | N | Freq (%) | P | (%) |
| 1. Low risk | 43 | 42.2 | 8 | 18.6 | 0.1 | 3 | 7 | 0.7 | 93 |
| 2. Intermediate risk | 39 | 38.2 | 14 | 35.9 | 4 | 10.3 | 89.7 | ||
| 3. High risk | 20 | 19.6 | 8 | 40 | 1 | 5 | 95 | ||
N: Number. Freq: frequency, expressed in percentage
:Low risk versus intermediate and high risks, when associated to progression and related death
| Progression | Related death | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CLIPi score | Nº | Freq (%) | PR | P | 95% CI | Nº | Freq (%) | PR | P | 95% CI |
| 1. Low risk | 8 | 18.6 | 0.4 | 0.04 | 0.2 – 1.0 | 3 | 7 | 0.8 | 0.7 | 0.2 – 3.2 |
| 2. Interm. and high | 22 | 37.3 | 1.2 | 1.018 – 1.655 | 5 | 8.6 | 1.0 | 0.9 – 1.1 | ||
Interm. and high: Group combining intermediate and high risk. N: number. Freq: frequency, expressed in percentage. PR: prevalence ratio. 95% CI: 95% confidence interval
An observational, cross-sectional study was performed in order to evaluate the CLIPi score as a prognostic factor in patients with the diagnosis of early stage MF, seen at the Sector of Photodermatology at HUCFF/UFRJ.
The sample was made of 102 stage IA or IB MF patients, undergoing follow-up for at least 5 years. The majority was classified as low-risk according to the CLIPi score.
The main prognostic factor continues to be the staging according TNMB classification.5,14In this sample, only the presence of plaque lesions was associated to a higher frequency of stage progression. In a study being prepared for publication conducted by the same authors of this study, using the same cases, besides the presence of plaque lesions, disease involvement of more than 10% of the body surface area, abnormal lactic dehydrogenase and beta-2-microglobulin, besides stage IB itself, were identified as poor prognosis factors. Supporting these findings, advanced age at diagnosis, male gender, intense pruritus, lymph node enlargement, peripheral eosinophilia, the presence and the size of Pautrier’s microabscesses, folliculotropism, large cells suggestive of transformation on histology, immunohistochemistry evidencing loss of positivity of CD7 and CD5 in mature T-cells, positivity for CD4 and positivity for CD30 higher than 15% and, finally, detection of TCR clonality in peripheral blood were described as factors of worse prognosis.1,3,6,9,10,11,14,15
As previously described, to improve prognostic assessment Benton et al.suggested the prognostic score CLIPi. From their initial findings and validation in a cohort with a significant number of patients, the authors identified that, regarding early stage MF, there was a statistically significant difference in terms of disease progression and survival, when the patients were divided into three groups. Those classified as low-risk had a 10-year survival of 90.3%, and 84.5% did not show stage progression during that time. Those with intermediate risk had a 10-year survival of 76.2%, and 68.8% did not progress. Finally, high-risk patients had a 10-year survival of 48.9%, and 54.5% of progression-free survival. The CLIPi score as a prognostic tool was able to refine the evaluation and, therefore, was recommended by the authors, with the reservation that it should be tested in multicentric studies.12
This study found 42.2% low-risk, 38.2% intermediate-risk, and 19.6%, therefore minority, high-risk patients in a sample of 102 patients, according to the CLIPi score. When low, intermediate and high risk were compared to disease progression and subsequently to disease-related death, even though there was a higher percentage of progressing cases among those classified as high-risk, this difference was not statistically significant (p=0.1). In the same way, there was no difference between the groups regarding mortality. Paradoxically, the highest percentage of 5-year survival was among high-risk patients. Therefore, in our sample, this datum conflicted with what was proposed in the study by Benton et al.12
When the intermediate- and high-risk cases were grouped and compared to low-risk cases, a higher prevalence of stage progression was identified in the higher risk group, with an estimated prevalence ratio of 1.2-fold, and this difference was statistically significant (p=0.04). however, the confidence interval does not allow for substantiation of this hypothesis since it contains the value 1.0. Thus, in the present study there was a tendency of a higher prevalence of progression in patients with early stage MF who did not have a low risk CLIPi score. The same was not seen for the occurrence of death.
In 2015, Wernham et al.published the findings of a study with 86 early stage MF patients. The patients were divided into two groups: non-progression and progression of the stage (to at least IIB). CLIPi score was tested in a group with 60 patients, of which 30 had progressed. The 30 remaining patients, classified as non-progressing, were matched by age with the progression group. A higher percentage of low-risk patients was identified among those who did not progress, however, this difference was not statistically significant.11
Three other studies that analyzed the CLIPi score were published in 2016. Sanz-Bueno et al.studied the performance of the CLIPi prognostic tool in a cohort with 82 early stage MF patients. The 10-year survival found was of 86% for low-risk patients, 91% for intermediate-risk and 64% for high-risk. Similar to what we found in our study, there was also a paradoxical finding with those cases in that among with those with intermediate risk, there was a higher survival when compared to those with low risk. Progression-free survival in 10 years was of 77%, 74% and 69%, respectively. The differences, however, were not significant.16
Danish et al. published their results of the application of CLIPi in a cohort with 390 patients. Of those, 305 had early stage MF. Five-year survival was 98.4%, 88.2% and 60.8% for low-risk, intermediate-risk and high-risk patients, respectively. In the same way, disease-free survival after 5 years was 99.0%, 88.1% and 65.8%, respectively. In both outcomes, the difference between the groups was statistically significant (p<0.0001). The authors considered that the score had a good performance in terms of risk stratification for early stage MF patients, and as Benton et al., suggested its validation in appropriate prospective studies.17
Finally, Nikolaou et al. published their findings regarding the study of prognostic factors in a series of 393 patients with early stage MF. All 393 were classified according to the CLIPi score. The authors identified that, after 5 years, according to CLIPi, high-risk patients had a 4.19-fold risk of stage progression and 5.25-fold higher mortality risk, when compared to those with low risk, findings that showed a statistically significant p-value and adequate confidence interval. However, it was not possible to demonstrate a difference for the group with intermediate risk in comparison to that with low risk.14
We point out that it is crucial to advance in the prognostic evaluation of MF patients, since a small but significant percentage, even if classified as early stage, courses with disease progression, affecting survival.
ConclusionWe conclude that the results of the usage of the CLIPi score in our sample were conflicting, as demonstrated by the relevant literature, therefore requiring new studies for the definition of its applicability. It is important to highlight that the limitations of this study are its retrospective design and its relative small sample. It was mentioned in the studies consulted the initiative of a prospective, multicentric study in Europe, with the goal of analyzing the performance of PROCLIPi; apparently an adjustment to improve accuracy of CLIPi score. We suggest waiting for those publications.
AknowledgementsWe would like to thank Prof. Dr. Nurimar Conceição Fernandes by the thorough review.
Financial support: None.
Conflict of interest: None.