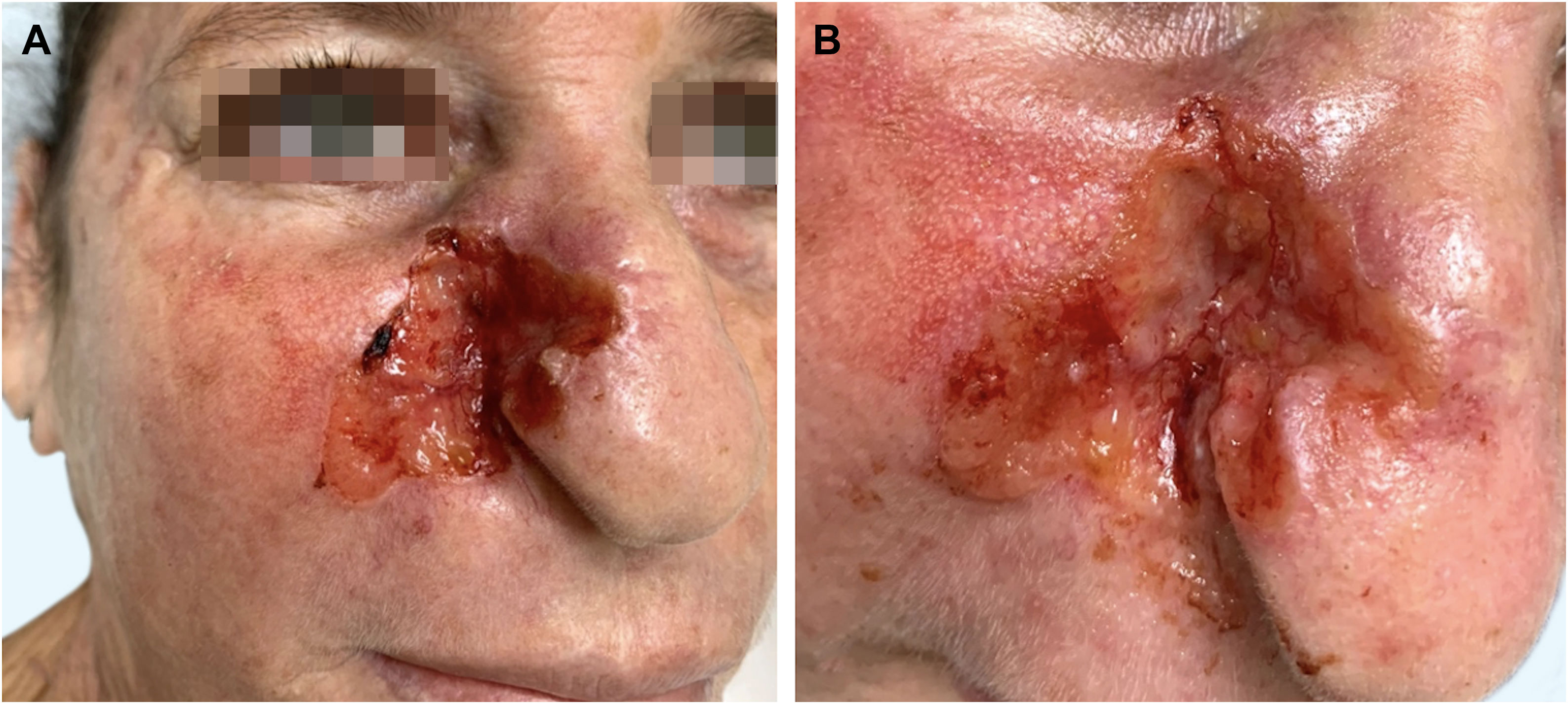
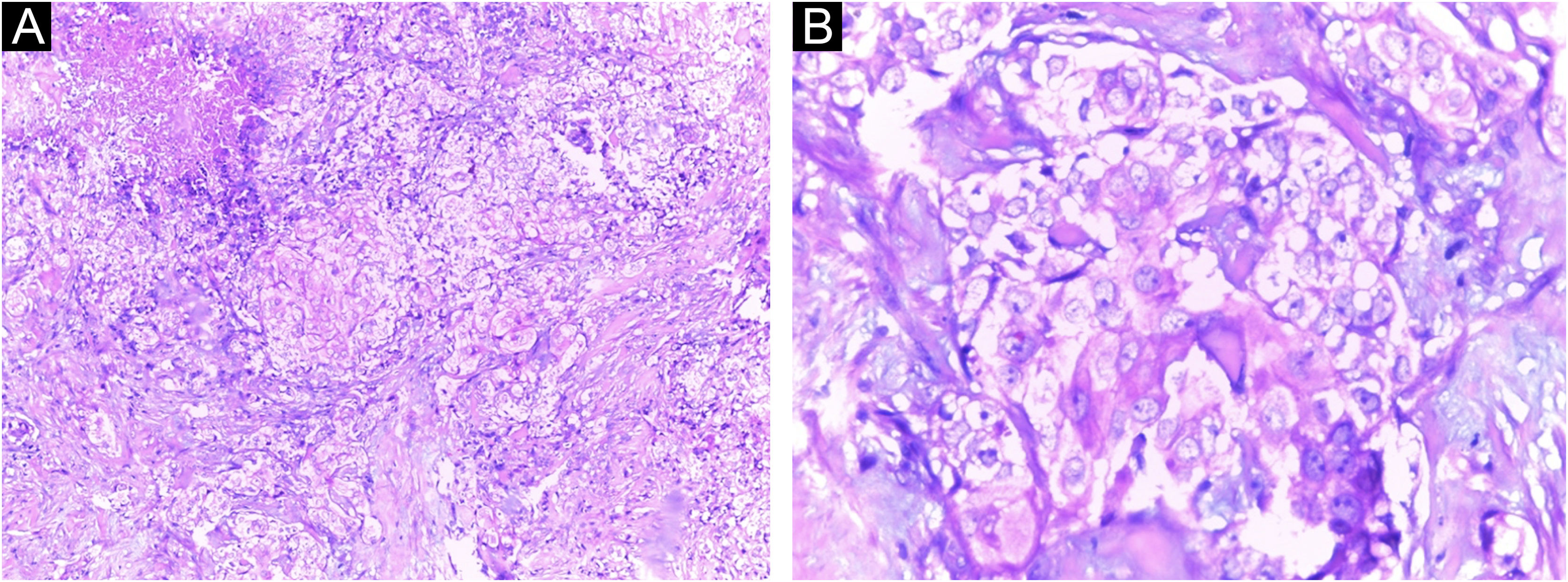
A Caucasian woman, 54 years old, Fitzpatrick phototype II, complained of a painful lesion on the right malar region, with rapid growth and aggressive behavior, which had appeared three months before. The patient lived in a rural area and worked in agriculture, referring chronic sun exposure due to her working conditions. When questioned, she denied history of trauma at the site of the lesion, previous skin cancer, immunosuppression, and exposure to artificial radiation. Dermatological examination showed an infiltrated and painful erythematous plaque with exudation, crusts, ulceration, and telangiectasias in the malar region extending to the right nasal ala (Fig. 1). Skin damage due to chronic sun exposure was also identified, manifesting as xerosis, melanoses, solar elastosis, actinic keratoses, and loss of skin elasticity on the face, anterosuperior thoracic region, and upper limbs. The initial diagnostic hypotheses were terebrant basal cell carcinoma and invasive squamous cell carcinoma. An incisional biopsy of the lesion was performed and histopathology showed a malignant epithelial proliferation with islands of clear cells and areas of necrosis; in addition to some cells with vacuolated cytoplasm, suggestive of a sebaceous neoplasia (Fig. 2). Rare keratinization foci were identified, suggesting squamous cell carcinoma (Fig. 3). Periodic acid Schiff (PAS) staining with and without diastase revealed the presence of cytoplasmic glycogen, excluding the possibility of sebaceous differentiation.
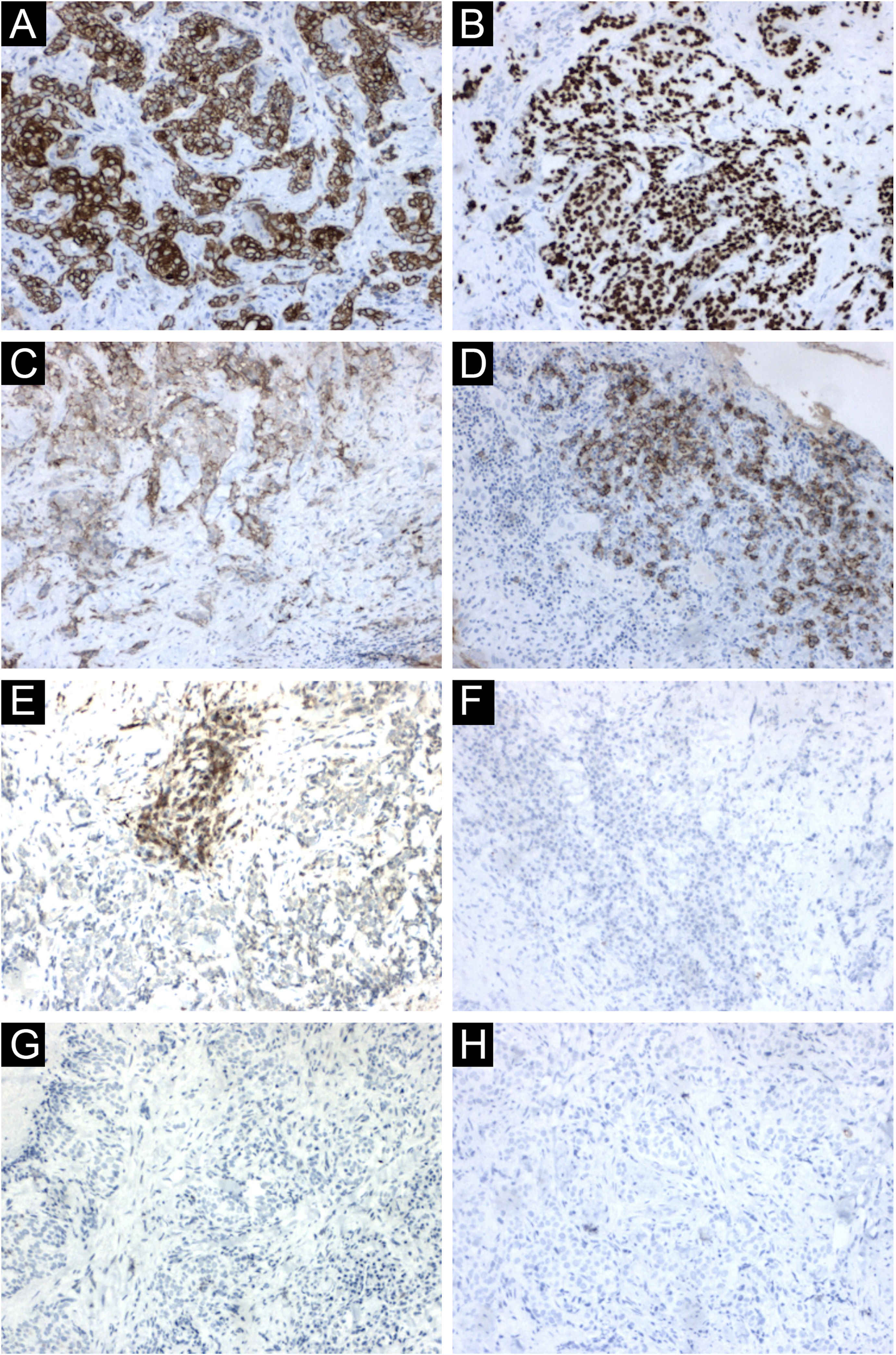
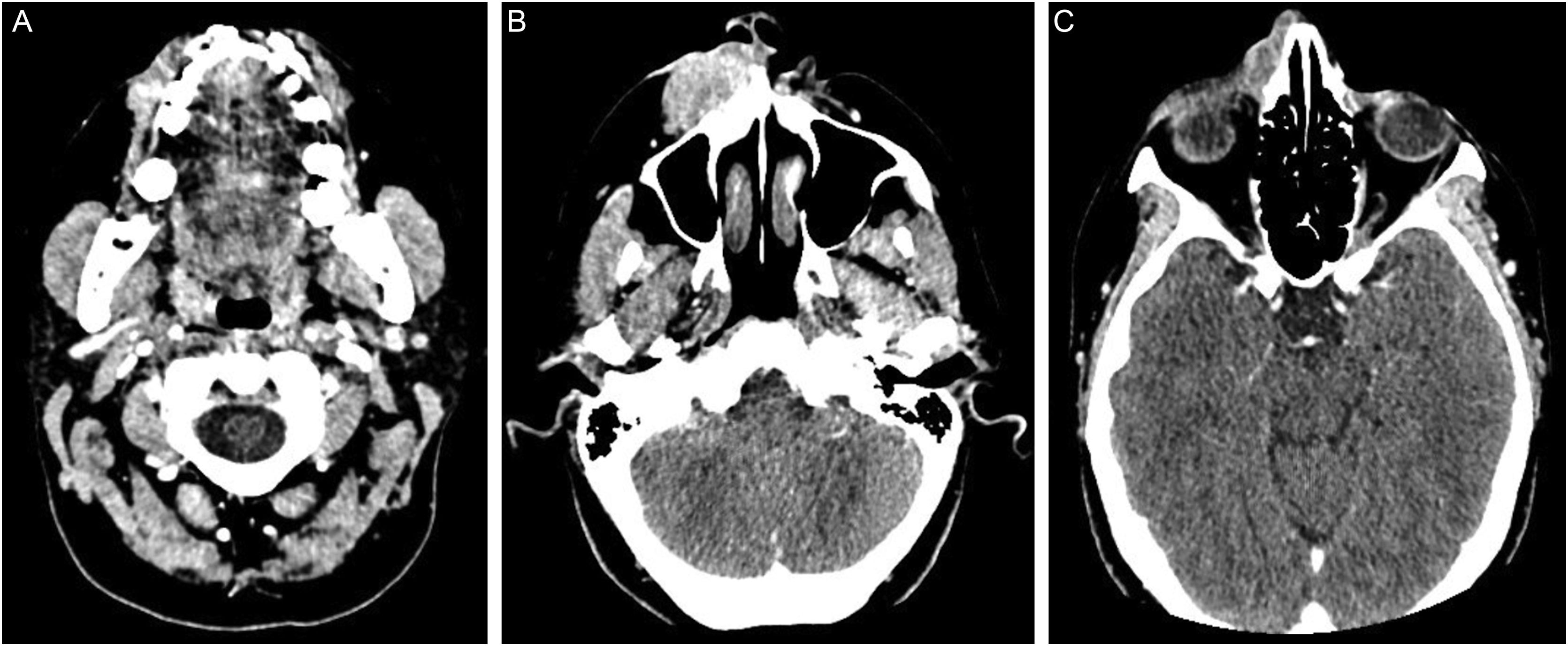
The immunohistochemical analysis showed positivity for cytokeratin 5/6 and p63; and was focally positive for BerEp4, EMA, and factor XIIIa; with negativity for adipophilin, androgen receptor and CEA (Fig. 4), confirming the squamous origin of the neoplasm. Face and neck computed tomography showed a solid expansive lesion with extensive superficial ulceration and intense heterogeneous contrast enhancement, extending to the right nasal ala and maxillary region. Nasal and right maxillary sinus bone destruction was observed, close to the right orbit and the dental root of the upper right canine (Fig. 5). No metastases were found in the imaging studies. Considering the extent of the lesion, the oncology and head and neck surgery team opted for neoadjuvant chemotherapy and subsequent evaluation of a surgical approach.
Immunohistochemical findings. (A) Neoplastic cells immunoreactive with cytokeratin 5/6. (B) Neoplastic cells immunoreactive with p63. (C) Focal positivity for BerEp4. (D) Focal positivity for EMA. (E) Focal positivity for factor XIIIa. (F) Neoplastic cells non reactive with adipophilin, (G) with androgen receptor (H) and with CEA
Computed tomography of the face and neck: (A) proximity of the neoplasm to the dental root of the upper right canine. (B) solid expansive lesion with wide superficial ulceration, extending to the nasal ala and right maxillary region, measuring 3.6 × 1.9 × 4.5 cm. (C) nasal bone destruction and upper extension, close to the right orbit. The tumor shows marked and heterogeneous contrast enhancement
Cutaneous squamous cell carcinoma (SCC) is classified into many subtypes, with a wide range of clinical manifestations ranging from indolent growth to aggressive tumors with significant metastatic potential.1 The undetermined category includes signet-ring SCC, follicular SCC, papillary SCC, SCC arising in adnexal cysts, squamoid eccrine ductal carcinoma, and SCC with clear-cell differentiation.1
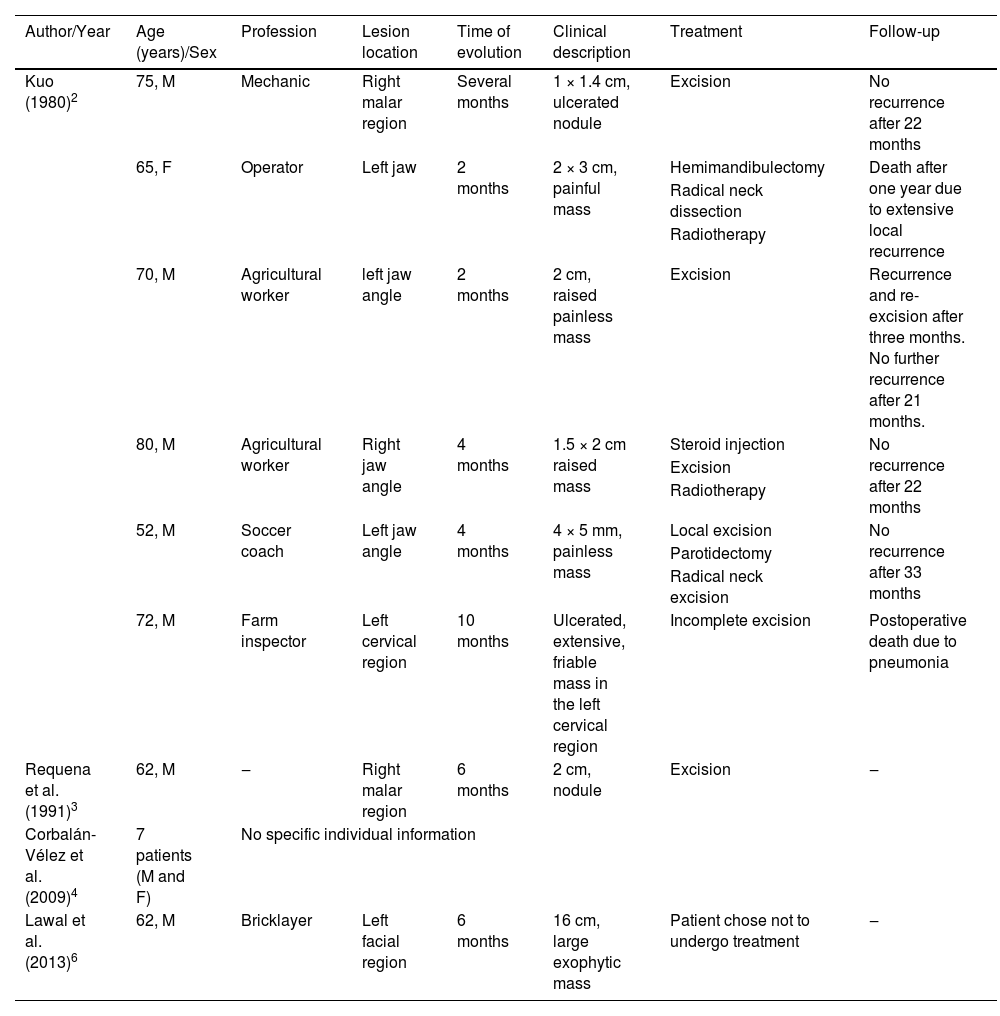
Cutaneous clear-cell SCC is a rare neoplasia the etiology of which is still incompletely understood. This variant was described in 1980 by Kuo.2 Since then, around ten articles have been published about this neoplasm,2–9 none of them in Brazil (Table 1).
Summary of published cases of invasive cutaneous squamous cell carcinoma with clear cell differentiation
| Author/Year | Age (years)/Sex | Profession | Lesion location | Time of evolution | Clinical description | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|
| Kuo (1980)2 | 75, M | Mechanic | Right malar region | Several months | 1 × 1.4 cm, ulcerated nodule | Excision | No recurrence after 22 months |
| 65, F | Operator | Left jaw | 2 months | 2 × 3 cm, painful mass | Hemimandibulectomy | Death after one year due to extensive local recurrence | |
| Radical neck dissection | |||||||
| Radiotherapy | |||||||
| 70, M | Agricultural worker | left jaw angle | 2 months | 2 cm, raised painless mass | Excision | Recurrence and re-excision after three months. No further recurrence after 21 months. | |
| 80, M | Agricultural worker | Right jaw angle | 4 months | 1.5 × 2 cm raised mass | Steroid injection | No recurrence after 22 months | |
| Excision | |||||||
| Radiotherapy | |||||||
| 52, M | Soccer coach | Left jaw angle | 4 months | 4 × 5 mm, painless mass | Local excision | No recurrence after 33 months | |
| Parotidectomy | |||||||
| Radical neck excision | |||||||
| 72, M | Farm inspector | Left cervical region | 10 months | Ulcerated, extensive, friable mass in the left cervical region | Incomplete excision | Postoperative death due to pneumonia | |
| Requena et al. (1991)3 | 62, M | ‒ | Right malar region | 6 months | 2 cm, nodule | Excision | ‒ |
| Corbalán-Vélez et al. (2009)4 | 7 patients (M and F) | No specific individual information | |||||
| Lawal et al. (2013)6 | 62, M | Bricklayer | Left facial region | 6 months | 16 cm, large exophytic mass | Patient chose not to undergo treatment | ‒ |
F, Female; M, Male.
SCC usually manifests itself on photo exposed areas of elderly Caucasian men with a history of chronic sun exposure and multiple skin neoplasms.2,6,8 Most articles in the literature describe head and neck lesions, some of which with rapid growth.8 In the present case, a rapid-growing lesion with aggressive behavior was identified on the face of a 54-year-old woman, with a history of chronic sun exposure and no previous skin neoplasia.
Clear-cell SCC can be classified into three variants: keratinizing (type I), non-keratinizing with no connection to the epidermis (type II), and pleomorphic (type III).2 The histopathological findings of the present report are consistent with the keratinizing variant (type I) according to Kuo.2 In 2007, a new classification was proposed according to the number of clear cells in the anatomopathological evaluation: cases with ≥80% of clear cells represent clear-cell SCC; cases with <80% and ≥50% are defined as SCC with marked clear cell change; and cases with <50% and >10% are described as SCC with moderate clear cell change.5 According to this classification, the neoplasm described in the present case can be identified as SCC with marked clear cell change.
On histopathology, the atypical clear cells showed evident nuclear pleomorphism, squamous differentiation foci, and areas of acantholysis, with some dyskeratotic cells amidst pseudo glandular spaces.1 The main differential diagnosis is sebaceous carcinoma, which is characterized by vacuolated, lipid-containing cytoplasmic cells positive for factor XIIIa, EMA, adipophilin, androgen receptor, AE1/AE3 cytokeratins, and perilipin on immunohistochemical analysis.1,10 In the present case report, the immunohistochemical analysis disclosed marked positivity for cytokeratin 5/6 and p63, with focal positivity for BerEp4, EMA, and factor XIIIa. Adipophilin and androgen receptors were non reactive (Fig. 4). Other differential diagnoses include trichilemmoma, clear cell acanthoma, pilar tumor, balloon cell nevus, balloon cell melanoma, and renal metastatic carcinoma.6 Mohs micrographic surgery is the treatment of choice for head and neck cutaneous SCC.
It is difficult to define the prognosis of this rare variant, as few cases were published in the literature up to 2022. Cohen et al. (2008) suggested that human papillomavirus (HPV) infection may be associated with tumor oncogenesis, but only two reported cases were associated with this virus.9 New studies are required to elucidate the behavior of clear cell SCC.
In conclusion, the present study describes the atypical case of a 54-year-old woman with a facial neoplasm compatible with clear cell SCC, histopathologically mimicking sebaceous carcinoma. Both dermatologists and dermatopathologists must be aware of the peculiarities of this SCC variant, which grew fast and was quite aggressive in the present report.
Financial supportNone declared.
Authors' contributionsEmily Neves Souza: Design and planning of the study; drafting and editing of the manuscript; collection, analysis, and interpretation of data; critical review of the literature; critical review of the manuscript.
Lucia Martins Diniz: Design and planning of the study; effective participation in research orientation; intellectual participation in the propaedeutic and/or therapeutic conduct of the studied case; critical review of the literature; critical review of the manuscript; approval of the final version of the manuscript.
Luana Amaral de Moura: Design and planning of the study; drafting and editing of the manuscript; collection, analysis, and interpretation of data; critical review of the literature.
Alexandre Calegari Oliosi: Design and planning of the study; drafting and editing of the manuscript; collection, analysis, and interpretation of data; critical review of the literature.
Marcela Scárdua Sabbagh de Azevedo: Design and planning of the study; drafting and editing of the manuscript; collection, analysis, and interpretation of data; critical review of the literature.
Márgya Neves Souza: Design and planning of the study; drafting and editing of the manuscript; collection, analysis, and interpretation of data; critical review of the literature.
Conflicts of interestNone declared.
Study conducted at the Dermatology Outpatient Clinic, Hospital Universitário Cassiano Antônio Moraes, Universidade Federal do Espírito, Vitória, ES, Brazil.