Desmoplastic melanoma, a distinct and uncommon variant, is characterized as an invasive lesion with proliferation of fusiform melanocytes in the dermis and subcutaneous tissue, associated with varying patterns of desmoplasia. Neurotropism and neural differentiation may occur. The clinical presentation is variable and nonspecific, easily confused with other fibrous neoplasms. The disease is locally aggressive and shows lower metastasis rates than other types of melanoma. Histopathology may be insufficient, requiring positive immunohistochemistry for S-100 protein and other antigens of melanocytic differentiation. Because desmoplastic melanoma represents a true clinical, dermoscopic, and histopathological diagnostic challenge, a case of invasive desmoplastic melanoma is reported, affecting a photoexposed area in an elderly woman after histological revisions and an initial diagnosis of fibroma.
Desmoplastic melanoma (DM) is a rare variant, representing less than 4% of cutaneous melanomas, with a global incidence of two cases per one million inhabitants.1-4 Since its description in 1971 by Conley et al. until a survey in 2009, approximately 1,100 cases were published worldwide.5
DM is a fibrous tumor, characterized histologically by the proliferation of fusiform melanocytes in the dermis, with variable degrees of stromal collagen deposits (desmoplasia).1-4 The neoplastic cells are frequently depigmented and display mild to severe nuclear atypia, sometimes with neurotropism and neural differentiation.1-4 Immunohistochemistry is positive for protein S-100, the most useful marker for diagnosis of DM due to its high sensitivity, with nuclear and cytoplasmic marking in 97% to 100% of cases.1-6
The clinical presentation is nonspecific, characterized by a firm amelanotic papule or nodule on a photoexposed area, especially the head and neck in older men. Diagnosis is prone to error due to the similarity to other fibrous lesions. DM displays a particular behavior and calls for a distinct surgical approach.1,2,5,6
We report a challenging clinical case of invasive DM in which the clinical presentation, nonspecific initial histopathological analysis, and initial diagnosis of fibroma resulted in diagnostic delay and an unfavorable outcome.
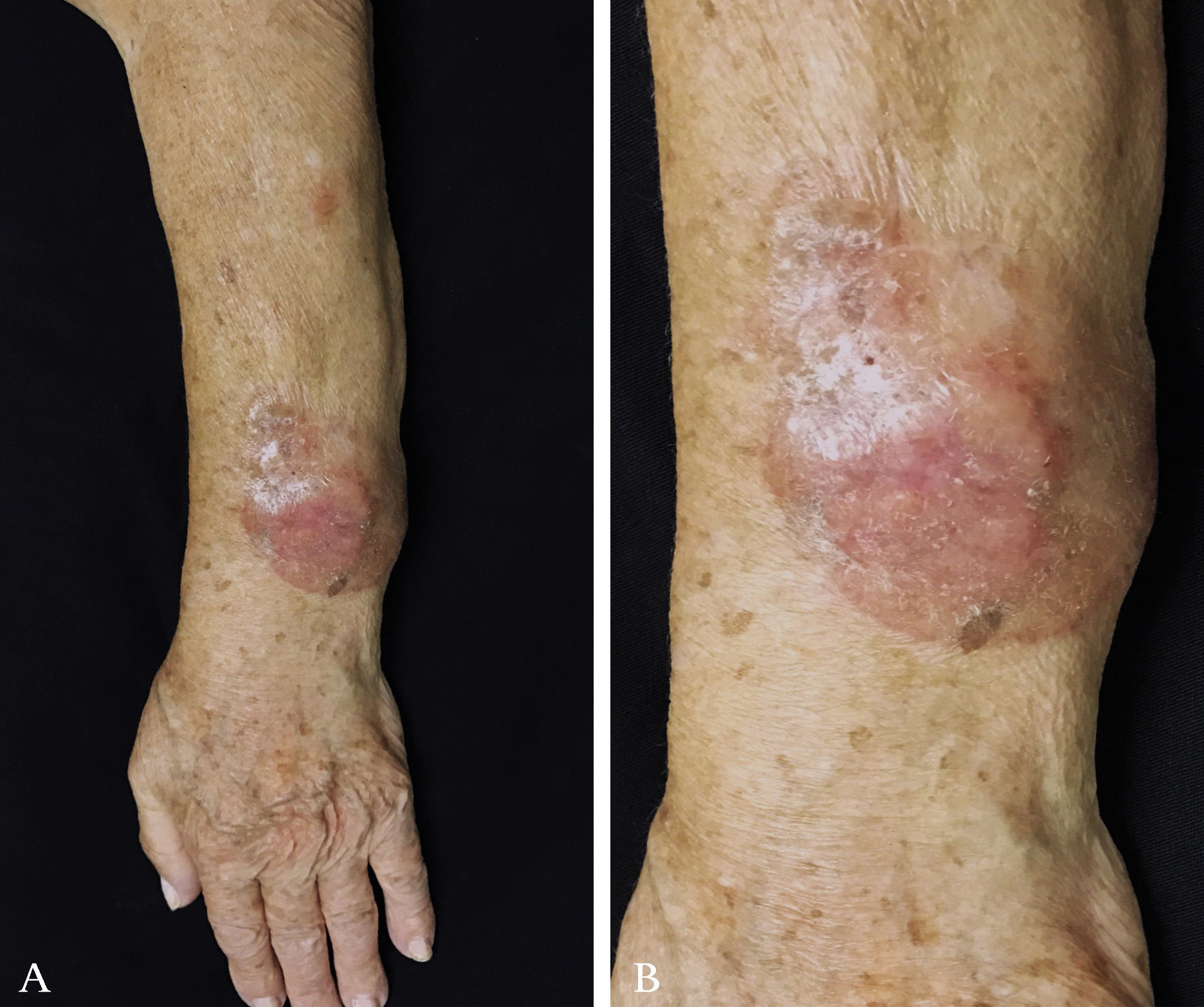
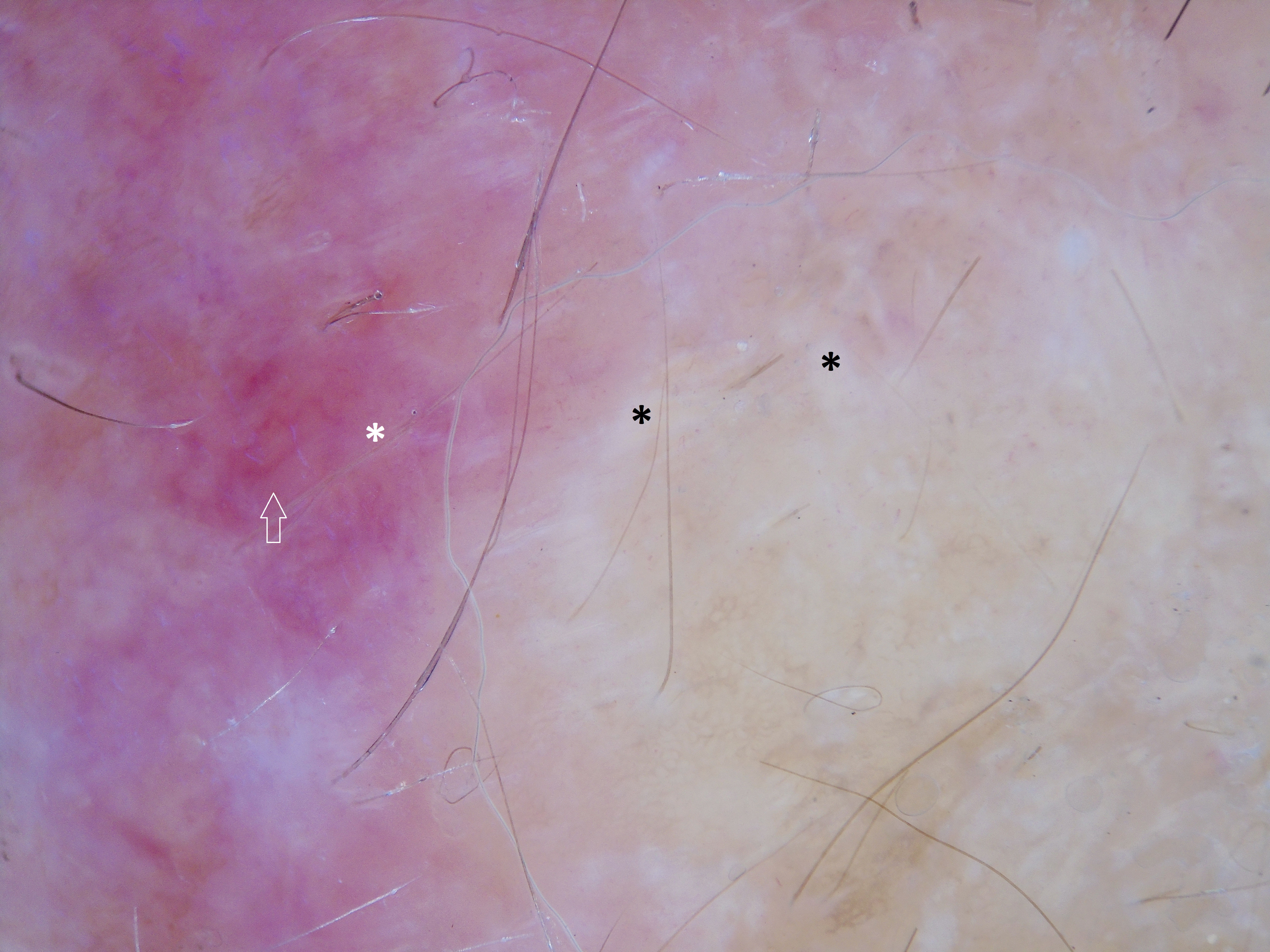
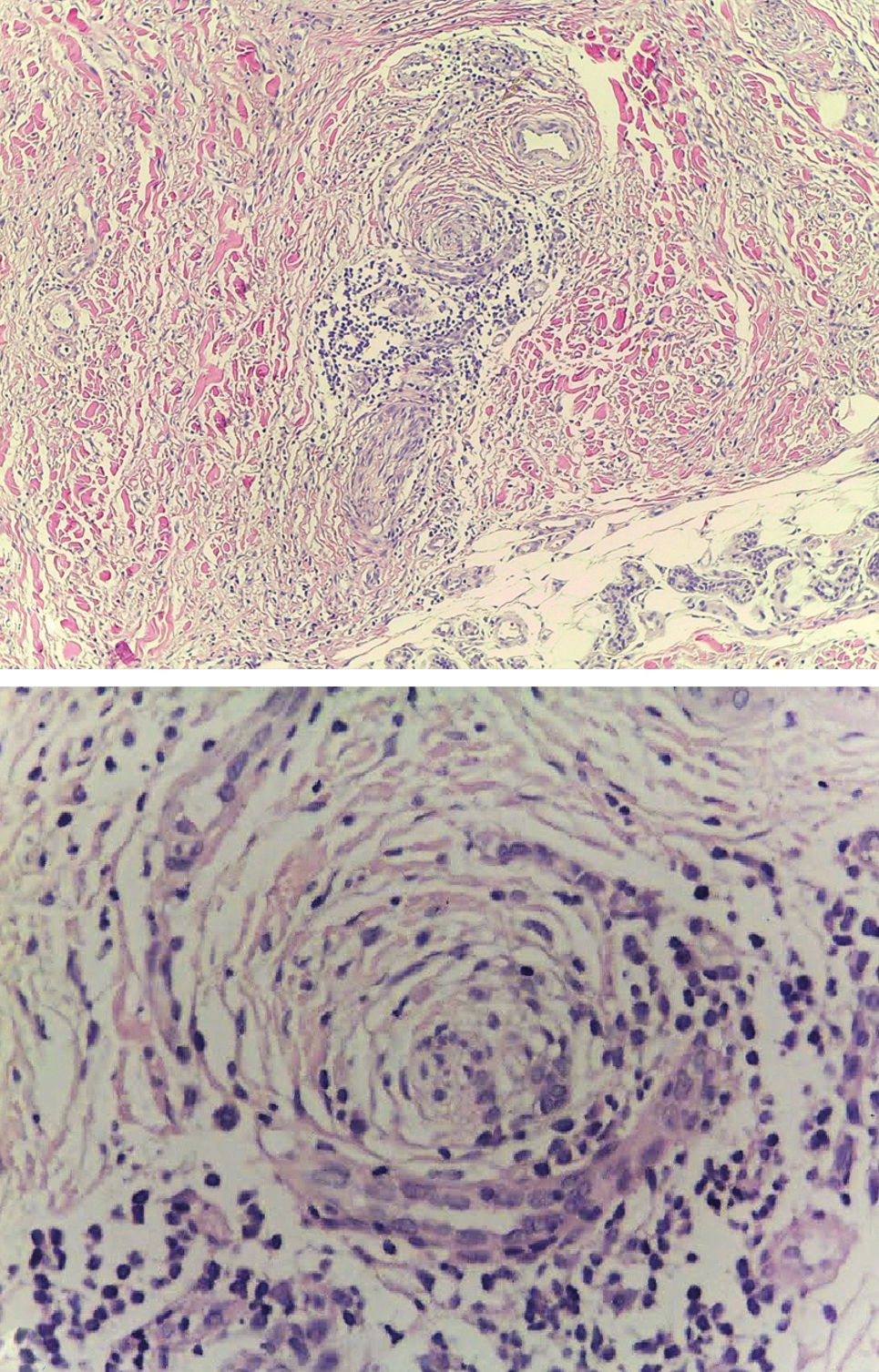
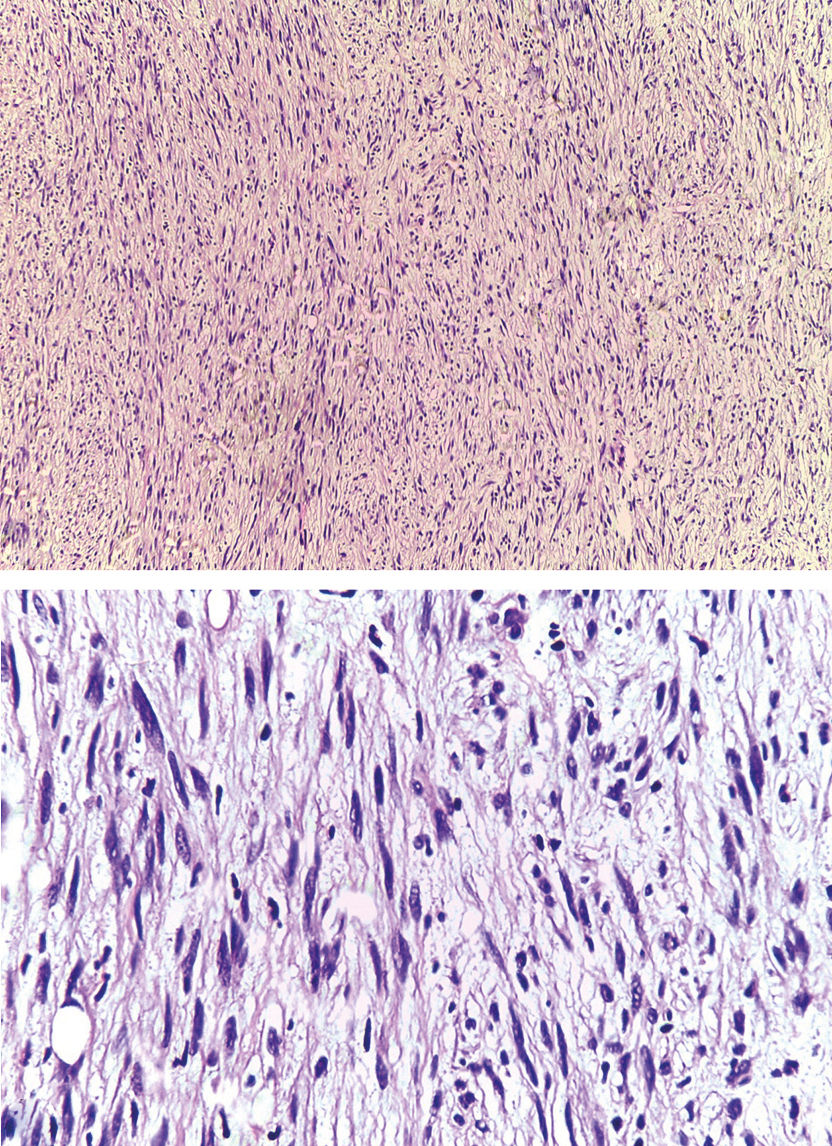
Case ReportA 79-year-old woman, white, appeared at the Dermatology Service due to the appearance of a nodule on her left forearm. In 2011, at another hospital, she underwent excision of a nodule on the same site, with a histopathological diagnosis of fibroma. Upon dermatological examination in 2016, she presented a painless erythematous tumor on her left forearm, with a stony consistency, adhered to deep planes, have grown steadily for a year, leading to reduced strength in the ipsilateral upper limb (Figure 1). Dermoscopy showed polymorphous vessels and milky-red and whitish areas, with a cicatricial appearance (Figure 2). Ultrasound of the lesion showed a solid expansive formation extending to the ulna, causing pressure on underlying myotendinous structures. Histopathology of the lesion revealed infiltration by a neoplasm consisting of fusiform cells with sparse cytoplasm and elongated nuclei, with moderate pleomorphism and permeated by collagenized stroma (Figure 3). Since the clinical picture is nonspecific, successive revisions of slides were performed, complemented by immunohistochemistry, which was diffusely positive for S-100 protein and SOX10 but negative for melan-A and HMB-45, thus characterizing DM.
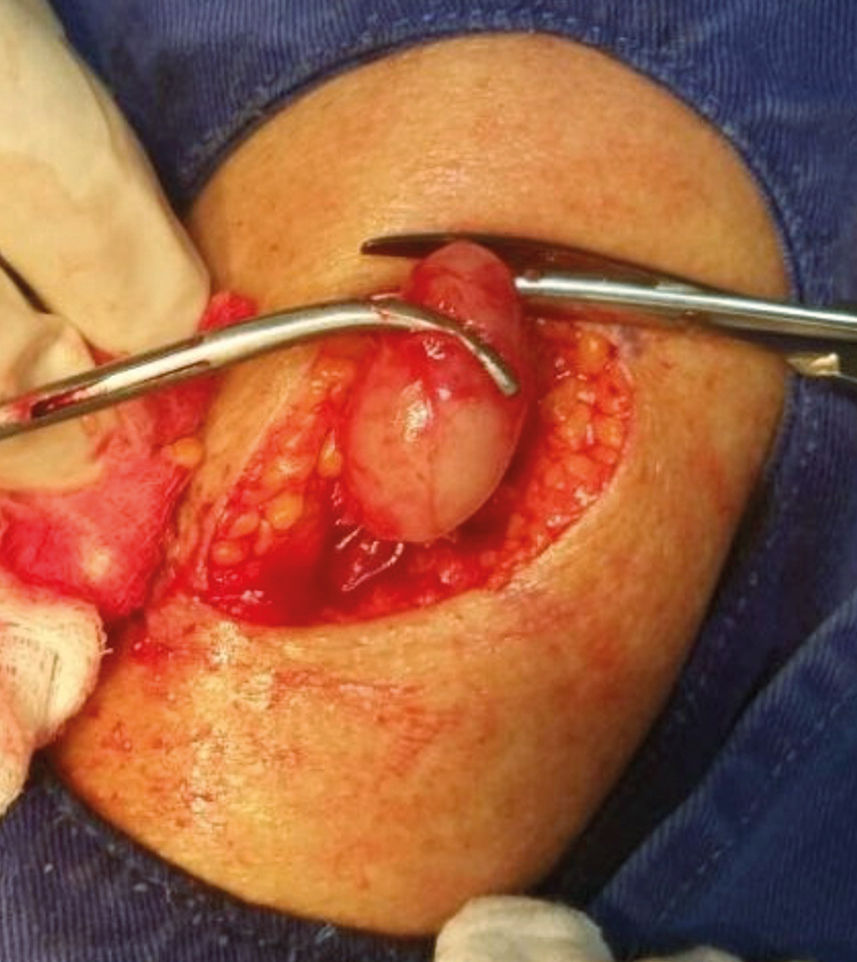
In addition to this lesion, examination evidenced another mobile, painless nodule on the left arm, which had appeared in the previous six months (Figure 4). Histopathology of this nodule showed a spindle cell neoplasm with neural differentiation, consistent with metastasis to the subcutaneous tissue, favoring the diagnosis of invasive DM (Figure 5). Patient underwent amputation of the left forearm due to the infiltration of deep structures. There was no evidence of distant metastases on investigation with imaging tests (computerized tomography and scintigraphy).
DiscussionDesmoplastic melanoma, a rare and distinct variant, can appear as a new lesion (15% to 20%) or in association with other subtypes of melanoma, most frequently lentigo maligna (42% to 56%).4,7 The clinical presentation is highly variable and nonspecific, generally characterized by a papule, nodule, or amelanotic plaque (44.3% to 73%) with a firm, fibrous consistency, extending from the dermis to the subcutaneous tissue, as in the lesion in the case reported here.1-6
Diagnostic delay or even error is not uncommon, due to the similarity between DM and other malignant lesions (e.g. carcinoma and fibrosarcoma), or as in the case reported here, with benign lesions (fibromatosis, dermatofibroma, and melanocytic nevus).2–4
DM occurs predominantly in men (2:1) of older age (mean 66 years). Chronic sun exposure explains the predilection for photoexposed areas: head and neck (51%), extremities (30%), and trunk (17%).1,2 The case described here was a woman with both the most frequent age bracket and location (a photoexposed area).1
Due to the amelanotic appearance of DM, dermoscopic findings are based on the vascular pattern, with irregular linear and dotted vessels and whitish areas with a cicatricial appearance, as in the dermoscopy in this case, and “peppering”.8,9
Histopathology shows an infiltrate of atypical and fusiform melanocytes, producing or releasing collagen, leading to a dense fibrous matrix. The tumor is highly infiltrative, invading the dermis and subcutaneous tissue, and displays variable patterns of desmoplasia, neurotropism, and neural differentiation.2-4 As in the case reported here, hematoxylin-eosin staining may be insufficient for the diagnosis due to the depigmentation of the neoplastic cells, thus requiring immunohistochemistry.1-5 IHC is positive for S-100 protein, the most sensitive reaction,3 and is generally negative for the other melanocytic differentiation antigens like melan-A and HMB-45, as in our case.1-5
Based on the degree of desmoplasia, DM has been classified in two forms: pure and mixed. The mixed form shows less than 90% desmoplastic involvement, high mitotic index, higher frequency of regional lymph node involvement, higher recurrence rate, and thus worse prognosis.1-4
Unlike non-desmoplastic melanomas, lymphatic metastasis is uncommon (zero to 18.8%), but the tumor is highly infiltrative and locally aggressive, consistent with the evolution in the case reported here, of a relapsing nature1. Tumors that display neurotropism show higher Clark levels (IV and V), mitotic activity, and local recurrence.1,2
Treatment is essentially surgical, with excision of the lesion as early as possible. In the neurotropic subtypes and in lesions with thickness up to 2mm, the excision should be performed with a margin of at least 1cm to 2cm, while in larger lesions the margin should be more than 2cm.1,2,6 The role of sentinel lymph node biopsy is not well established, and some authors propose indications such as: Breslow index greater than 1cm, Clark level V, mixed subtype, and presence of neurotropism, ulceration, and high mitotic rate.1,2,6,10 Adjuvant radiotherapy provides benefit in cases of local recurrence, excision with narrow margins, residual tumors, and neural involvement.1,2
Distant metastases (lungs, liver, bones) were observed in 7% to 44% of DM, more commonly in the mixed form; therapeutic options exist in these cases, still without proof, such as ipilimumab and vemurafenib.1,2
Overall five-year survival for DM patients varies from 67% to 89%; advanced age, male sex, and location of the lesion on the head and neck are associated with increased risk of death.1
Financial support: None.
Conflict of interest: None.