Dear Editor,
Urticaria is a common skin condition, affecting 15% to 20% of the population at least once in their lifetime.1 This condition has many presentations and triggers, and is commonly associated to drug reactions. The wheal is a dermatological elementary lesion, with three typical features: central edema, surrounded by reflex erythema; pruritus; and evanescent nature.1 A relevant aspect is the color of the wheal. Typically, wheals induced by histamine are light-colored, surrounded by a pink erythema that disappears with pressure. Wheals with intense purplish or violaceous indicate significant vascular damage and plasma leakage, such as in urticarial vasculitis.1 Lesions tend to appear suddenly after a trigger — via immunological, infectious, physical or idiopathic mechanisms —, disappearing in less than 24 hours.2
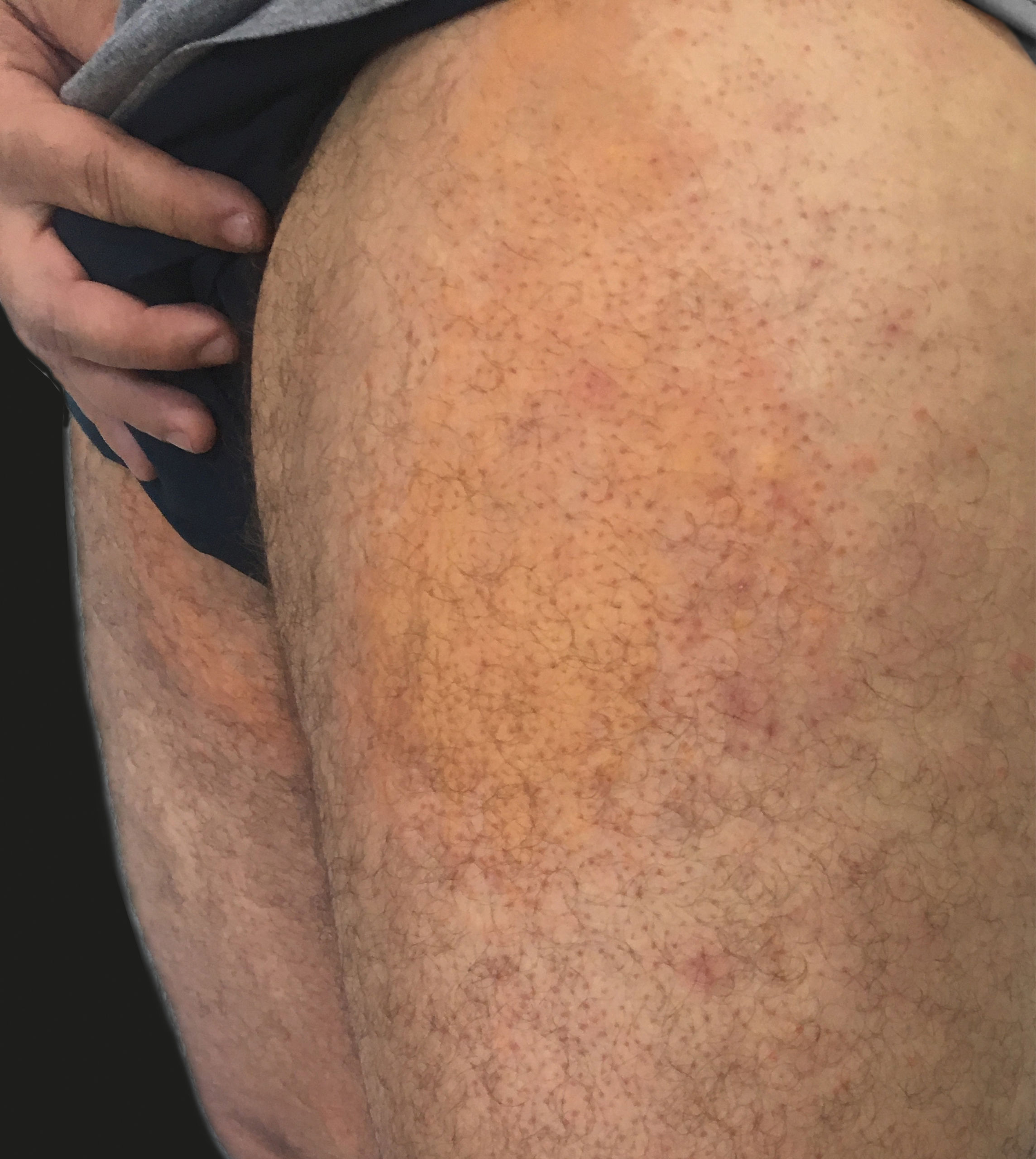
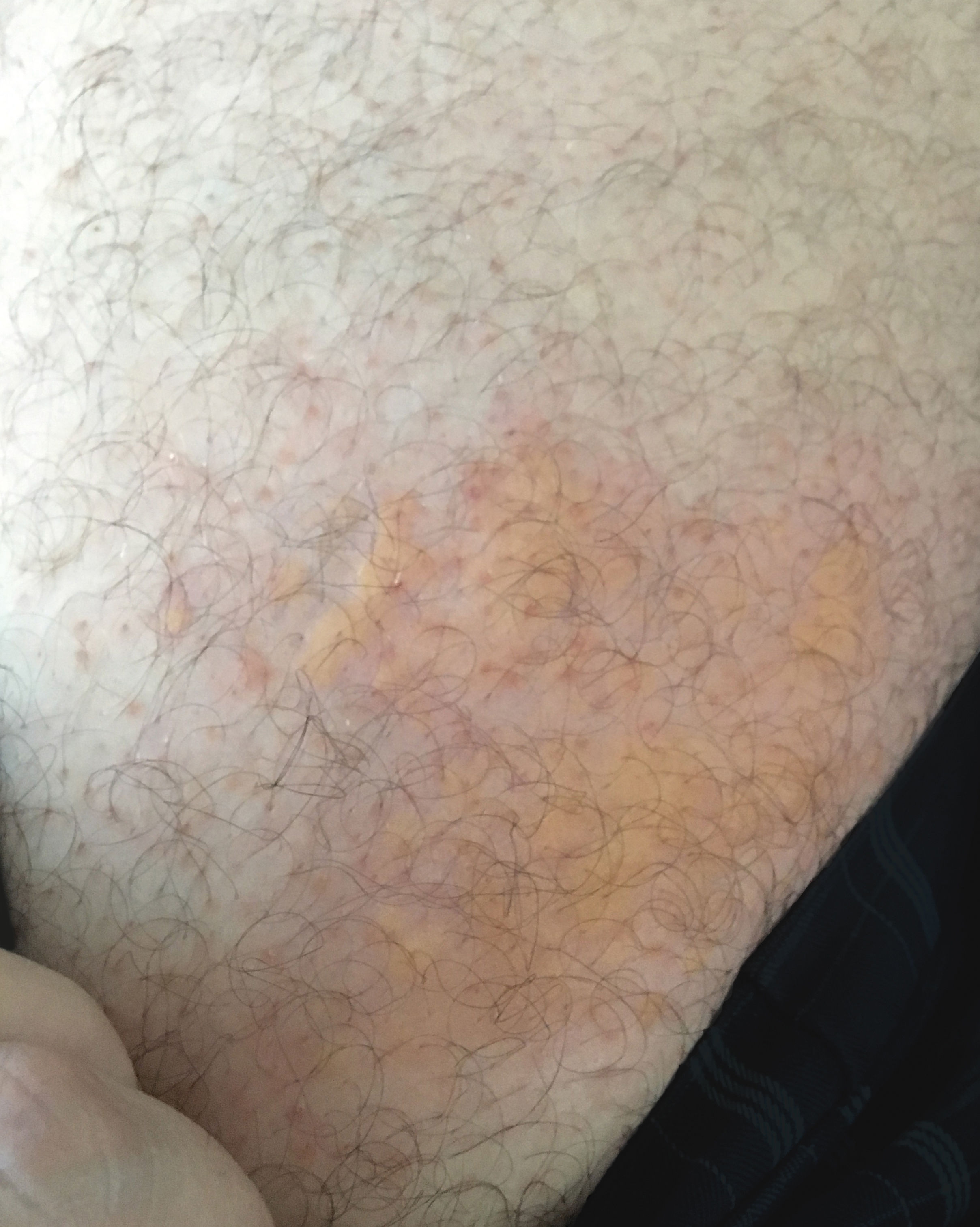
It is believed that the color of yellow urticaria (YU) is due to exudation and buildup of bilirubin pigment in the dermis, associated to an increased permeability of the blood vessels. Therefore, this variant has a systemic repercussion, with diagnostic importance for hyperbilirubinemia.2 YU was reported for the first time in 2002 by Patel & Mutasim as one of the rarest forms of presentation of the disease. Except for the yellow color, its features are similar to those of the classic form – evanescent, pruritic and edematous.3
Unlike the wheals that resolve in 24 hours, jaundice can persist for days due to the affinity of the bilirubin to the elastin found in the skin.3
The differential diagnoses include xanthomas, cutaneous pigmentation caused by drugs and metals, and excessive ingestion of foods containing carotene.3 The evanescence, the rapid onset after introducing the medication, the pruritus, and the good response to antihistamines confirm the diagnosis.
YU is associated to liver diseases that present with hyperbilirubinemia.3 Hepatitis, cirrhosis, acute liver failure, and liver tumors are some of the diseases reported in patients with the condition.2–5 There is no report of YU triggered by the use of antiretrovirals.
In view of the unusual presentation of the condition and its systemic implications, we report a case of YU after institution of prophylactic antiretroviral therapy (ART) with atazanavir, ritonavir, tenofovir and lamivudine. It is worth highlighting that this is the first case of YU associated to ART.
A 35-year-old man started prophylactic ART after occupational injury with a HIV-positive patient. He was prescribed atazanavir, ritonavir, lamivudine, and tenofovir for 30 days. On the second day of treatment, he developed asymptomatic jaundice. On the 16th day, edematous papules and plaques appeared, which were itchy and yellowish with an erythematous halo, disappearing with pressure (Figures 1 and 2), distributed on the trunk and limbs. The lesions were recurrent and lasted for less than 1 hour. Laboratory exams revealed hyperbilirubinemia due to indirect bilirubin (7.3mg/dL), with a total bilirubin of 7.5mg/dL (normal up to 1mg/dl); hemoglobin: 15g/dL; hematocrit: 42%; leukocytes: 5700/mm3; aspartate-aminotransferase: 19 U/L (normal up to 37 U/L); and alanine-transaminase: 28 U/L (normal up to 78 U/L). Lactic desidrogenase, amylase, alkaline phosphatase and gamaglutamyltransferase were normal. Fexofenadine (120mg per day) was prescribed and kept up to the conclusion of ART treatment, with subsequent disappearance of the lesions. Of note, hepatitis B and C virus and HIV serologies were negative.
The combination of antivirals described is well known and considered tolerable, with low levels of discontinuation, and efficacy rates that vary according to the type of injury. Hyperbilirubinemia, with or without transaminases elevation, is a common side effect of ART, via direct hepatotoxicity (common), interference in the conjugation of bilirubin or hemolysis (rare). Protease inhibitors, particularly ritonavir and atazanavir, which were used by the patient, are capable of competitively inhibiting the enzymes responsible for the conjugation of bilirubin in liver tissue, increasing serum levels of the non-conjugated fraction.
Although there were no transaminase abnormalities suggestive of liver damage, the patient had an elevation of the indirect bilirubin, resulting from the action of the drug in the conjugation process and its excretion.
The clinical picture of the patient is similar to other reports. The lesions appeared after initiation of prophylactic ART and subsided with the use of antihistamine. The antihistamine was maintained during all 30 days of antiretroviral treatment, with complete resolution of the condition. It is worth remembering that treatment for the yellow variant and for common urticaria is the same, with the use of first- or second-generation antihistamines.
Knowledge about YU is still extremely poor with scarce publications. In view of the importance of the lesions for the diagnosis of hyperbilirubinemia and the increasing use of antiretrovirals, knowledge and recognition of possible adverse reactions becomes necessary.
Financial support: None.
Conflict of Interests: None.