The recently published 4th Edition of the World Health Organization Classification of Head and Neck Tumors addresses the most relevant and updated aspects of tumor biology, including clinical presentation, histopathology, immunohistochemistry, and prognosis of head and neck tumors. The objective of the present study is to compare these updates to the 3rd edition of that book with regard to mucosal melanomas and to highlight the potential factors that differ those tumors from cutaneous melanomas. We observed progress in the understanding of oral and sinonasal mucosal melanomas, which also present themselves, in the molecular scope, differently form cutaneous melanomas.
The recently published 4th edition of the World Health Organization (WHO) Classification of Head and Neck Tumors approaches the new knowledge acquired in the area – especially in the field of molecular pathology – since its last publication in 2005. The Blue Books, as they are Known in the scientific community in allusion to the color of the cover page, are the world reference to tumor classification for pathologists, clinical practitioners, surgeons, and oncologists. These books bring the most up to date information about etiology, epidemiology, histological classification, immunohistochemistry, molecular pathology, prognosis, and differential diagnoses of neoplasms.
Approximately 15-20% of all melanomas occur on the head and neck, 80% of which have a cutaneous origin, and the remaining majority, an ocular origin.1 However, mucosal melanomas should not be forgotten. They represent 0.7-3.8% of all melanomas, being considered completely different from other cutaneous variants in relation to clinical, embryological, genetic, and prognostic aspects.1-3
In decreasing order of frequency, head and neck mucosal melanomas involve the sinonasal cavity, oral cavity, pharynx, and larynx.2,3 Unfortunately, the possible etiological factors remain unknown.2,3,4
Classical parameters to the diagnosis of mucosal melanomas such as Clark’s level and Breslow’s depth have no confirmed application to mucosal melanomas.2,3 Difficult visualization of the lesions and rich vascularization may be possible explanations for worse mortality rates in cases of mucosal melanoma when compared to cutaneous melanomas.4 Perhaps, due to the greater aggressiveness of this mucosal tumor, in the AJCC 7th edition Cancer Staging Manual, all head and neck mucosal melanomas are staged as T3-4.5
For the Brazilian population, data from the National Cancer Institute did not make reference to mucosal melanomas in the estimates for the year 2016.6 In a study conducted in the state of Rio Grande do Norte, mucosal melanomas corresponded to approximately 11% of all cases of melanoma predominantly affecting the head and neck (76.1%).7 We emphasize that the histopathological differentiation between a primary mucosal melanoma and a metastasis of an unknown or regressed cutaneous tumor can be challenging,4 which requires a close correlation with a detailed clinical history. In this review, we will cover the main aspects of the new classification, in comparison to the previous publication and the current literature.
Melanoma of the Oral CavityMucosal melanomas of the oral cavity represent approximately 50% of all head and neck mucosal melanomas and about 0.5% of oral malignancies, with a questionable male predominance and frequent lymph node metastases or distant metastases. In Japan, the oral cavity is reported as a common site of melanoma.1
Histologically, mucosal melanomas of the oral cavity have a radial growth phase and a vertical growth phase, but the classification into subtypes as it is performed in cutaneous tumors into subtypes does not apply as it is performed in cutaneous tumors. No pagetoid spread is observed, and, more often, the tumor is diagnosed in the invasive phase. In most cases, melanic pigment is easily detected by the Fontana-Masson stain. Mitosis is rare, and the superficial epithelium is usually atrophic. Assessing the depth of the invasion (Breslow and Clark) has limited application, perhaps because most tumors are deeper than 4mm when diagnosed. Factors associated with worse prognosis include tumor polymorphism, vascular invasion, advanced age, and necrosis.1
Melanomas of the oral cavity are associated with KIT, RAS, and BRAF mutations, in decreasing order of frequency.2 Interestingly, there are indications that up to one-third of the cases are preceded by mucosal melanosis and, when compared to cutaneous tumors, a greater number of amelanotic cases is observed.4
Mucosal Melanoma of the Nasal CavityThe chapter that addresses tumors of the nasal cavity introduced mucosal melanoma, which corresponds to approximately 1% of all melanoma cases. This type of melanoma does not show gender predilection. The peak of incidence occurs in the seventh decade. The lesions are generally polypoid with high mitotic index. More than 50% of cases are amelanotic. S100 protein and melanocytic markers (HMB45, melan-A, tyrosinase, MITF, and SOX10) show variable sensitivity with higher positivity rates in epithelioid cases and lower rates in fusocellular cases.2
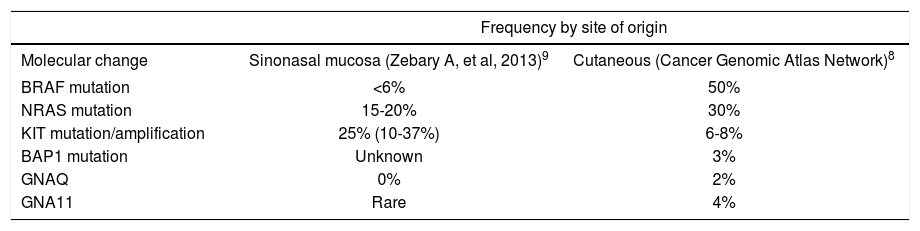
Metastatic disease and advanced age are considered factors associated with worse prognosis. Table 1 shows the most frequent mutations in cutaneous melanomas and sinonasal mucosal melanomas.2,8,9
Variation of molecular changes in melanomas by site of origin
| Frequency by site of origin | ||
|---|---|---|
| Molecular change | Sinonasal mucosa (Zebary A, et al, 2013)9 | Cutaneous (Cancer Genomic Atlas Network)8 |
| BRAF mutation | <6% | 50% |
| NRAS mutation | 15-20% | 30% |
| KIT mutation/amplification | 25% (10-37%) | 6-8% |
| BAP1 mutation | Unknown | 3% |
| GNAQ | 0% | 2% |
| GNA11 | Rare | 4% |
Partial copy of the table available in the WHO Classification of Head and Neck Tumors, 4th Edition. El-Naggar AK et al., 20172
Mucosal Melanomas of the larynx are extremely rare. They most commonly affect men, and the most frequently reported subsite is the supraglottic larynx. Histologically, it resembles melanomas on other anatomical sites. Since normal melanocytes can be located in the submucosal compartment, the junctional component is not indispensable for its diagnosis, and surgical excision is the treatment of choice.1
The prognosis is poor with high metastasis rates for regional lymph nodes and distant organs.1 Since the aim of the 4th Edition was to incorporate advances in the biology of head and neck tumors in a reasonable pattern and focused on everyday practice, some tumors such as melanoma of the larynx were excluded.10
Financial support: None.
Conflict of interest: None.