Recent studies that investigated the effect of vitamin D on skin cancer risk have exhibited inconsistent results.
Objective:The aim of the study was to evaluate vitamin D status in patients with actinic keratosis.
Methods:A cross-sectional study was conducted on 31 patients with actinic keratosis and 29 healthy controls. Serum vitamin D levels in the study group were determined by liquid chromatography/tandem mass spectrometry.
Results:Serum 25(OH)D levels in patients with actinic keratosis were significantly higher than those of the healthy controls (P=0.04). Prevalence of 25(OH)D deficiency was significantly higher in the healthy controls (75.9%) compared to the patients with actinic keratosis (54.8%), but the difference was not statistically significant (P= 0.09).
Study limitations:The cross-sectional design of the study, data on smoking based on patient self-report, and subjects’ different dietary habits, which can influence 25(OH)D levels, are the study’s limitations.
Conclusion:Serum vitamin D level can be used as a marker for ultraviolet B radiation from sun exposure; therefore, it can be used in individuals at risk of actinic keratosis. Oral intake of vitamin D through diet or supplements is proposed instead of prolonged ultraviolet exposure to maintain adequate vitamin D serum levels. Further research is needed to elucidate the role of vitamin D in skin carcinogenesis
The main source of vitamin D is endogenous synthesis in the skin, induced by ultraviolet B (UVB) radiation.1-4 The main circulating form of vitamin D, 25-hydroxyvitamin D [25(OH)D], has a half-life of approximately 2 weeks and is a better reflection of all sources of vitamin D exposure and status than 1,25(OH)2D3[4].The same spectrum of UV radiation (280–320nm) is the main cause of skin cancer, but at the same time it is required for vitamin D synthesis in the skin.5-8 Studies in the literature have shown different results, with some reporting an association between lower vitamin D levels and others reporting higher vitamin D levels and increased skin cancer risk.5,9-11
Actinic keratosis (AK), also known as solar keratosis, is a pre-cancerous epidermal lesion caused by long-term exposure to UV radiation, which may progress to squamous cell carcinoma (SCC). In humans, vitamin D receptor (VDR) polymorphisms are associated with the increased development of solar keratoses.12 Another study reported that VDR knockout mice developed numerous non-melanoma skin cancers (NMSCs).13,14
The aim of this study was to evaluate vitamin D status in patients with AK.
MethodsPatients and controlsThis cross-sectional study included 31 patients with AK and 29 healthy controls, all with a normal body mass index (BMI; 18–25kg/m2), as it has been shown that vitamin D deficiency is associated with obesity.15 All individuals were studied during the same period (21 November 2014 to 21 March 2015) to avoid seasonal variations in vitamin D levels and were selected from the metropolitan area of Istanbul, Turkey. All participants were white-skinned. Sun exposure was similar between patients and controls.
Patients with a history of systemic treatment (e.g. corticosteroids, immunosuppressive therapy, vitamin D or calcium supplementation, cholesterol-lowering drugs) or phototherapy within 6 months of the study were excluded. Subjects with a history of diabetes mellitus, parathyroid or thyroid disorders, autoimmune diseases, anemia, atopy, chronic renal or liver disease, malignancy, plus currently pregnant or lactating females, smokers, and subjects applying sunscreen were also excluded. Data on smoking relied on self-reports. Informed consent was obtained from all individual participants included in the study. The study was approved by the Şişli Hamidiye Etfal Training and Research Hospital Ethics Committee (approval no: 409, date: 23/12/2014) and was conducted according to the Declaration of Helsinki principles.
Serum vitamin D analysisThe serum concentration of 25(OH)D was determined for each participant on the day of enrolment by using liquid chromatography/tandem mass spectrometry (Quattro Premier XE; Waters Corporation, Milford, MA, USA). Serum 25(OH)D concentrations ≤20 ng/mL were defined as deficient.16
Statistical analysesStatistical analyses were carried out using the Statistical Package for Social Sciences (SPSS) version 15 (IBM, Armonk, NY, U.S.A.). The Mann-Whitney U test was used for the statistical analysis, and the results were expressed as mean ± standard deviation (SD). Correlations were performed by Spearman’s correlation analysis. P < 0.05 was considered significant.
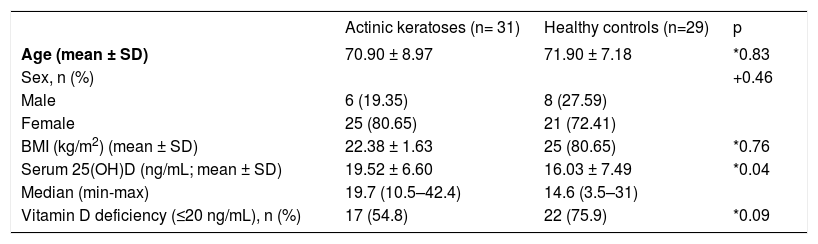
ResultsGender ratio, age, and BMI did not differ significantly between patients with AK (25 women, 6 men; mean ± SD age 70.90 ± 8.97 years; BMI 22.38 ± 1.63) and healthy controls (21 women, 8 men; mean ± SD age 71.90 ± 7.18; BMI 22.37 ± 1.68) (P= 0.46, P= 0.83, P= 0.76, respectively) (Table 1).
Demographic and clinical characteristics of subjects
| Actinic keratoses (n= 31) | Healthy controls (n=29) | p | |
|---|---|---|---|
| Age (mean ± SD) | 70.90 ± 8.97 | 71.90 ± 7.18 | *0.83 |
| Sex, n (%) | +0.46 | ||
| Male | 6 (19.35) | 8 (27.59) | |
| Female | 25 (80.65) | 21 (72.41) | |
| BMI (kg/m2) (mean ± SD) | 22.38 ± 1.63 | 25 (80.65) | *0.76 |
| Serum 25(OH)D (ng/mL; mean ± SD) | 19.52 ± 6.60 | 16.03 ± 7.49 | *0.04 |
| Median (min-max) | 19.7 (10.5–42.4) | 14.6 (3.5–31) | |
| Vitamin D deficiency (≤20 ng/mL), n (%) | 17 (54.8) | 22 (75.9) | *0.09 |
The mean ± SD serum 25(OH)D concentration of patients with AK (19.52 ± 6.60ng/mL) was significantly higher than that of healthy controls (16.03 ± 7.49ng/mL) (P= 0.04). A 25(OH)D deficiency (≤20 ng/mL) was observed in 54.8% of the patients with AK and 75.9% of healthy controls, but the difference was not statistically significant (P= 0.09) (Table 1).
No correlation between the number of lesions and serum 25(OH)D levels was found in the AK patients (P= 0.14; r= 0.268). When female and male proportions in the study groups were investigated, 25(OH)D levels were not found to be statistically more significant in female AK patients than in male AK patients (mean ± SD ng/mL; 19.82 ± 11.88 vs 19.45 ± 6.60; P= 0.38). Also, there was no significant correlation between age and 25(OH)D levels in AK patients and controls (P= 0.09, P= 0.87, respectively).
DiscussionUV radiation promotes skin cancer development by inducing photodamage in human keratinocytes through the formation of cyclobutane pyrimidine dimers (CPD), local immunosuppression, and the oxidative stress mechanism.17-21 Vitamin D may have a protective effect against UV radiation in vitro by reducing CPD, improving CPD repair, reducing UVB-induced apoptosis, and inducing the antioxidant metallothionein.6,22-24 But the photoprotective effect of vitamin D on keratinocytes may be dose-dependent.21,23,24
Non-melanoma skin cancers include AKs, Bowen’s disease, SCC, and basal cell carcinoma (BCC). The role of vitamin D in skin cancer is more complex than in other cancers. UV radiation is the main source of cutaneous vitamin D production and is the main etiologic factor for NMSC. This dilemma has led to inconsistent findings in the literature regarding the amount of sun exposure and vitamin D supplementation for skin cancer prevention.25 Furthermore, vitamin D levels in patients with NMSC have shown contradictory results. A recent study by Liang et al.26 found elevated 25(OH)D levels to be associated with increased BCC and SCC incidences. Van der Pols et al.27 evaluated the association between serum 25(OH)D levels and skin cancer risk in 1,191 adults. In subjects with a history of skin cancer, 25(OH)D levels above 75nmol/L were associated with a reduced incidence of SCC and with an increased incidence of BCC and melanoma. Eide et al.8 showed that a serum 25(OH)D level of 15ng/mL or higher was associated with an increased NMSC risk in 3,223 white-skinned patients enrolled in a health maintenance organization, but this was not statistically significant. In addition, a large prospective cohort study reported that elevated plasma 25(OH)D levels were associated with an increased risk of NMSC and melanoma in the general population.28 Tang et al.10 revealed that baseline 25(OH)D levels >75nmol/L were associated with a decreased risk of NMSC in elderly men.
In our study, mean serum vitamin D in patients with AK (19.52 ± 6.60ng/mL) was higher than in healthy controls (16.03 ± 7.49ng/mL). We are of the opinion that plasma 25(OH)D can be used as a marker for UVB radiation from sun exposure; therefore, it can be used to identify persons at risk of AK, as previously indicated by Afzal et al.28
In the literature, vitamin D deficiency has been reported to be very common in the elderly population. A recent Indian study revealed that 91.2% of the population over 50 years of age had vitamin D deficiency.29 In a study from the Robert Koch Institute, vitamin D deficiency was evident in 75% of elderly women between 65 and 79 years of age.30 AK is more prevalent in the elderly population. The fact that our population consisted mainly of elderly people may explain the lower than expected levels in the whole cohort.
To our knowledge, this is the first study to compare vitamin D levels in AK patients with healthy controls. However, there are several limitations in our study. It had a cross-sectional design, data on smoking relied on patient self-reporting, and variations in patients’ dietary habits may have influenced the levels of 25(OH)D. In addition, the study population was very small, and the findings should be viewed as preliminary.
ConclusionIn conclusion, we think that vitamin D can serve as a marker of chronic sun exposure; therefore, it may correlate with higher incidence of AK, although it does not increase the risk itself. In contrast, vitamin D provides well-established benefits, including protection against several cancers and fracture risk in the elderly. Therefore, regarding the recent studies showing that sun protective measures have not been found to contribute to vitamin D deficiency or insufficiency, we support the use of sun protection, see UV radiation as the main risk factor for AK, and recommend that an adequate amount of vitamin D should be obtained from vitamin D-enriched foods and/or from vitamin D supplements.31-33