Pentavalent antimonials remain as the standard drugs in the treatment of cutaneous leishmaniosis. The high cost, difficult administration, long treatment time, toxicity and increasing morbidity are factors that limit the use of these drugs.
Objectives:To describe the response to radiofrequency thermotherapy in the treatment of localized cutaneous leishmaniasis in Brazil, and to evaluate its safety and tolerability.
Methods:We conducted a non-comparative open trial with a total of 15 patients confirmed to have cutaneous leishmaniasis on parasitological examination. A single radiofrequency thermotherapy session at 50°C for 30 seconds was applied to the lesion and its edges. In patients with more than one lesion, only the largest one was treated initially. If after 30 days there was no evidence of healing, the smaller lesion was also treated with thermotherapy. Clinical cure was defined as visible healing for three months after treatment. The patients were followed-up for six months and there was no follow-up loss.
Results:Of all 23 lesions, only two evolved to complete healing without the need of treatment. Of 21 lesions, 18 (85.7%) achieved full healing. The main observed side effects were itching, burning sensation, pain and blisters.
Study limitations:Sample with a small number of patients and short follow-up.
Conclusion:Thermotherapy can be considered a therapeutic alternative in localized cutaneous leishmaniasis, especially in cases of single cutaneous lesions and with formal contraindications to conventional treatment with pentavalent antimonials.
Cutaneous leishmaniasis (CL) is an infectious disease caused by protozoa of the genus Leishmania, transmitted to men and other mammals through the bite of phlebotomine sandflies. The disease is endemic in more than 70 countries, with 90% of cases occurring in Afghanistan, Algeria, Brazil, Pakistan, Peru, Saudi Arabia and Syria. In the New World, it is found from the south of the United States to the north of Argentina. The annual incidence is estimated in 1-1.5 million cases.1,2 In Brazil, it is present in all states, with an increased incidence over the last 20 years, with a mean of 25,000 new cases/year and 8 to 18 cases/100,000 inhabitants. The North region contributes with the larger number of cases (around 40% of the total of registered cases) and has the highest mean coefficients (73.3 cases/100,000 inhabit.), followed by the Mid-West (35.4 cases/100,000 inhabit.) and Northeast (18.8 cases/100,000 inhabit.) regions.3 Although the state of Piauí, located in the Northeast of Brazil, is one of the states with the highest number of visceral leishmaniasis, its incidence of CL remains low, occupying the 4th place among the states of the Northeast. In 2014, the state of Piauí notified 85 cases of American tegumentary leishmaniasis, with a coefficient of detection of 4.1 cases per 100,000 inhabitants.4
Leishmaniases are among the most neglected diseases due to the limited resources invested in its diagnosis, treatment and control, together with its strong association to poverty and social conflicts. They occur more frequently in economically unfavored areas, without basic sanitation, low educational and income levels.5 They represent one of the dermatological conditions that most deserve attention due to their magnitude, by the risk of causing deformities in humans and also by the psychological involvement, that is reflected in the social and economic fields.2
In Brazil, the condition is also known as American tegumentary leishmaniasis (ATL), caused basically by three main species: Leishmania (Viannia) braziliensis, Leishmania (Viannia) guyanensis and Leishmania (Leishmania) amazonensis. However, most cases are caused by L. (V.) braziliensis, originating the main clinical form of ATL, localized CL. Other less common species causing the disease are L. (Viannia) lainsoni, L. (Viannia) naiffi, L. (Viannia) shawi and L. (Viannia) lindenbergi.3,6 The vectors are phlebotomine commonly known as sandflies, tatuquiras, birigui, with Lutzomyia flaviscutellata, L. whitmani, L. umbratilis, L. intermedia, L. wellcome e L. migonei being the main species involved in the transmission.7
Clinical manifestations of ATL are diverse, depending on the Leishmania species involved and host factors, including their immune response. It is also possible that these factors have a role in the persistence of the parasite.8 Clinically, ATL can present basically in 4 forms: localized CL, mucocutaneous leishmaniasis, disseminated CL and diffuse CL. The initial manifestation of the disease is characterized by an erythematous papule or nodule, usually on an exposed area of the skin, that progresses to a well-defined ulcer with rolled edges and coarse granulations in the center, covered by a serous-bloody exudate or not. They are usually found on exposed areas like the face, arms and legs.9 The involvement of nasal mucosa, palate, pharynx, larynx and vocal cords can be seen in up to 5% of patients. Regional lymph node enlargement, with or without lymphangitis, occurs in 12-30% of cases.10
Even though the diagnosis of CL can be made only by clinical-epidemiological criteria, ancillary tests are essential to differentiate the disease from other infectious, inflammatory and neoplastic dermatoses. Diagnosis is confirmed when the parasite is identified on direct examination, culture in specific media, skin histology or polymerase chain reaction (PCR).11 Montenegro intradermal test is an indirect method used to aid in the confirmation of the diagnosis.12
CL lesions can heal spontaneously, but that would take months to years. When not treated, complications such as disease extension into mucous membranes or disfiguring lesions can occur. This is particularly important in infections with the species belonging to the subgenus L. viannia (L. viannia braziliensis, L. viannia guyanensis and L. viannia panamensis), that have shown a strong tendency to systemic dissemination when compared to other species.13
Despite multiple efforts dedicated to the development of new drugs for the treatment of CL, pentavalent antimonials have been used for many decades as the drug of first choice. Commercially available are meglumine antimoniate (Glucantime® and sodium stibogluconate (Pentostam®.14 Brazil’s Ministry of Health recommends 10 to 20mg/kg/day of intravenous or intramuscular Glucantime®, during 20 days for the treatment of cutaneous forms. In case there is no complete healing three months after treatment, the regimen should be repeated for 30 days. If there is still lack of response, a second line drug should be used.3
One of the greatest problems regarding the use of pentavalent antimonials are their side effects. The main side effects include arthralgia, myalgia, anorexia, nausea, vomiting, postprandial fullness, epigastralgia, heartburn, abdominal pain, pruritus, fever, weakness, headache, dizziness, insomnia, palpitation, edema, hepatitis with increased transaminases and alkaline phosphatase, acute renal failure, pancreatitis and dose-dependent changes on electrocardiogram, such as alteration of the ventricular repolarization with ST inversion, prolongation of the QT interval, ischemic changes and bigeminy, polymorphic and polyfocal.15,16 Fatal arrhythmias are rare, with only a few cases of sudden death, likely related to ventricular arrhythmias.17 These reactions are more pronounced in the elderly and the drug should be used with caution in those older than 50 years old.18
Another disadvantage of this class of drugs is that they are contraindicated during pregnancy, lactation, individuals with hypersensitivity to the drug and patients with severe, chronic conditions (heart, liver, kidney diseases).14 The success rate with the described regimen is very variable in the literature, ranging from 60 to 90%, according to different authors and in studies conducted in different areas and services.16,19 It is worth highlighting that, even with the appropriate antimonial treatment, recurrences of mucosal involvement can occur in 2%, of treated cases and around 10% in untreated cases.10 Antimonial resistance by some Old World Leishmania species is another increasing problem, even though this has not been observed in Brazil.20 The prolonged course of treatment and the fact that the applications are multiple and painful, lead to the discontinuation of the treatment by the patient, possibly making the poor adherence one of the main causes for the appearance of strains resistant to the standard treatment.
The use of second line medications such as amphotericin B and pentamidine is indicated in cases of resistance or when meglumine antimoniate is unavailable.3 However, they have as limitations the high cost and the need of higher complexity services for the administration, as well as frequent and severe side effects.21,22
Historically, application of local heat has been used to treat CL lesions. In rural communities in South America and Africa, the empiric use of caustic materials such as silver nitrate, gunpowder, brown sugar, oil, battery solution or cauterization of lesions with a drop of candle wax or heated metal objects, such as forks and spoons is very common.23-25 In a study performed in rural areas of Ecuador, multiple alternative treatment methods were identified such as the application of caustics, petroleum products, heavy metals, veterinary products and preparations of plants and medicinal herbs. Many of these treatments were described by research participants as “hot “or “strong”, since they caused intense burning and pain. According to them, heating the lesions increased their chances of cure.26
Laboratory studies demonstrated that Leishmania species that cause cutaneous disease cannot multiply inside macrophages in temperatures above 39°C, and thermosensitivity is especially high for L. braziliensis and L. mexicana. These studies suggest that there is a growth and survival phase of these parasites that is sensitive to heat.27,28 These observations fostered the use of thermotherapy with hot baths, infrared light, direct electric stimulation, laser and photodynamic therapy for the treatment of CL lesions.29-33
Radiofrequency therapy has distinct advantages over other local methods of treatment. The heat produced by the radiofrequency waves penetrates uniformly to a depth of 4mm, in a way that the upper dermis, where are the amastigote forms of Leishmania, can be heated to high temperatures without damaging the surrounding skin. Besides, radiofrequency waves can be made by portable devices. The first study of its efficacy was performed in Guatemala with 66 randomized CL patients by L. braziliensis and L. mexicana and demonstrated a rate of cure of 73%, the same as patients treated with systemic pentavalent antimonial.34 However, in two randomized studies that also compared thermotherapy with systemic pentavalent antimonial performed in Colombia, the cure rates were lower, around 60% but still with the advantage of less costs and less side effects with radiofrequency thermotherapy. One of them randomized 292 patients with the diagnosis of CL by L. panamensis and L. braziliensis into two groups, the thermotherapy group and the systemic antimonial. In this study there was no statistically significant difference regarding the species in the response of the treatment used. The other randomized 130 with CL caused especially by L. guyanensis to receive conventional treatment and thermotherapy.35,36
Multiple randomized clinical trials compared thermotherapy to other therapeutic alternatives. A controlled randomized study performed in Afghanistan to treat CL by L. tropica with 401 patients, demonstrated 69.4% efficacy when compared to intralesional antimonial (75.3%) and intramuscular antimonial (44.8%).37 Later, a controlled randomized trial performed in Iran with 117 patients showed superiority of radiofrequency thermotherapy (80.7%) when compared to intralesional antimonial (55.3%).38 Another randomized controlled study treated CL by L. major in 54 American soldiers returning from Iran and Kuwait and achieved similar cure between thermotherapy (48%) and intravenous pentavalent antimonial (54%) but with much less side effects with thermotherapy.39 A recent study performed in India with 100 patients randomized into two groups showed an elevated efficacy of both the radiofrequency thermotherapy and the intralesional antimonial after 12 weeks follow-up.40 The efficacy and safety of miltefosine was compared to thermotherapy in a randomized controlled trial with 294 CL patients by L. panamensis and L. braziliensis performed in Colombia. There was no statistically significant difference between both treatments, which demonstrated a cure rate around 70%. There was also no statistically significant difference in the response to treatment regarding the causative species of the disease. However, the number of side effects reported with miltefosine was higher.41
In Brazil, a study performed in Bahia compared the systemic inflammatory response through the dosage of inflammatory cytokines after radiofrequency thermotherapy and after conventional treatment with pentavalent antimonial and demonstrated that, in both, there was an elevation in the inflammatory cytokines, with no statistically significant difference. However, it is not known if this change in the inflammatory response is a direct result of thermotherapy and the systemic medication or if it results from the healing process triggered by the treatments performed. It was also noted that in a patient with only one of two lesions treated with radiofrequency thermotherapy, the untreated lesion progressed to healing.42
This healing effect of the therapy in distant untreated lesions can be explained by systemic inflammatory changes that occur. After application of the heat therapy, there is a significant decline in the circulating T-cells and NK-like T-cells (natural killer-like), followed by an expansion of the NK cells (natural killer). A decline in the cellular proliferation of antigen-specific T CD4+ is also seen, as well as a depletion in the T CD8+ cells. Besides, the healing period was characterized by a decrease in the circulating regulatory T-cells, reduction in the production of IFN-γ and contraction in T helper CD4+ polyfunctional cells.43
Although the studies conducted exclude immunosuppressed patients, there is a case report of an HIV-positive patient with CL who did not respond well to the treatment with intralesional antimonial and was then treated with one session of radiofrequency thermotherapy with a good response, demonstrating that, in immunosuppression cases or in those with contraindications to the conventional treatment, thermotherapy can be a satisfactory alternative.44
To date, there are no approved vaccines against leishmaniasis and the drugs used for its treatment are extremely toxic, expensive and difficult to use. Therapeutic options for this disease remain very restricted. Moreover, there are evidences in the Old World of parasite resistance to the drugs commonly used, such as pentavalent antimonials. The search for new treatment options with few side effects, low toxicity, good tolerability, ease of use, with a reduced treatment time and low cost is necessary to spare individuals of the inconveniences of traditional therapies. The objective of this study is to describe the response to the treatment of CL with radiofrequency thermotherapy and evaluate safety and tolerability of thermotherapy for the treatment of CL.
MethodsA non-comparative open clinical trial was performed with 15 patients seen at the Hospital de Doenças Tropicais Natan Portella, a reference for infectious diseases in the state of Piauí, in some cities of the state of Maranhão and the Laboratório de Pesquisas em Leishmanioses (Lableish) in Teresina, Piauí, Brazil, from April to September 2015. All patients clinically suspicious for CL underwent ancillary tests to confirm the clinical diagnosis of CL. Montenegro skin test was performed on the medial surface of the forearm and the reaction measured 48 hours later. A biopsy of the lesion was also performed and the sample was used for histology, direct examination, culture and conventional PCR with the primers 150/152. When the lesions were secondarily infected, they were previously treated with cephalexin. All patients were tested for the human immunodeficiency virus and had general blood biochemistry.
Patients with the clinical-epidemiological diagnosis of CL, with a positive Montenegro test associated to at least one of the following ancillary tests were included in the study: demonstration of the parasite by direct observation in the lesion or with culture (medium Neal, Novy and Nicolle/NNN – modified agar blood), or by suggestive histology or by conventional PCR.
Exclusion criteria were the presence of lesions on the oral or nasal mucosae, presence of more than 10 cutaneous lesions, CL lesions within 2cm of the orbit, lesions on the nose, lips, eyes, urogenital or anal opening or adjacent to these sites and immunosuppressed patients.
After receiving detailed instructions about the study and the procedures performed, all patients or their guardians signed the consent form.
After disinfection of the lesions with iodine and local anesthesia with lidocaine 2% without vasoconstrictor, one session of treatment was performed using the portable radiofrequency device ThermoMed Model 1.8 (Thermosurgery Technologies, Inc., Phoenix, AZ, USA), according to the manufacturer’s instructions. Before the treatment, the tip of the device was applied to the border of the lesion, pointing to the center, heating it to a temperature of 50°C for 30 seconds and then the tip was moved to an adjacent portion until the lesion was completely covered. The size of the lesion was the determinant of the number of applications and the time spent in the session.
After selecting the desired temperature for the treatment (50° C), the device regulates the energy and maintains the selected temperature. Through a digital screen, it is possible to detect the temperature reached in the skin. The device emits a beep every two seconds and a louder sound after the selected time is finished, indicating that the treatment planned has come to an end, without the need of looking at the device. The application causes local erythema, therefore it is possible to identify which areas of the lesion were already treated and which were not. The device is light and uses rechargeable batteries, making it possible its use in rural areas or those with no electricity. It operated when the electrodes are applied on the skin, generating heat when the radiofrequency waves pass between two electrodes, reaching an area of about 3 x 4mm.
After the treatment, the patients were advised to cover the lesion with gauze and apply fusidic acid cream for seven days to avoid secondary infection. Patients were reviewed at 15 days, 30 days, 90 days, 180 days and 360 days. However, they were instructed to return in case of any complications. Photographs were taken before the treatment and in every follow-up.
When the patient had more than one lesion, the treatment was initially performed only in the larger one. If, after 30 days of the treatment, the remaining lesions did not show any signs of healing, radiofrequency thermotherapy was also performed on them.
Clinical cure was defined as complete epithelialization, total regression of the edema and erythema and visible scarring for three months after the treatment. Definitive cure was considered when there was no recurrence six months after the treatment. The total patient follow-up was of 12 months.
The patients that did not respond to this treatment were treated with meglumine antimoniate with the dose of 20mg/Sb+5/kg/day for 20 days, according to the recommendation of the Ministry of Health, Brazil.
Data collected were inserted into the program Microsoft Windows Excel 2010® and were processed and analyzed with the statistical program Stata/SE® 14.1 for Windows (College Station, Texas, USA), with which an analysis of frequency distribution and calculation of the 95% confidence interval were performed. The program Microsoft Windows Excel 2010® was also used for the tables and graphs.
ResultsA total of 15 patients participate in the study from April to September 2015. All patients had the diagnosis confirmed by at least one ancillary test performed. Demographic and clinical features of the patients are shown in table 1.
Demographic and clinical features of the patients (n = 15)
| Age (years) | Mean ± SD | 43.47 ± 20.97 |
|---|---|---|
| Sex | Male | 9 (60.00%) |
| Female | 6 (40.00%) | |
| No. of lesions | One lesion | 8 (53.33%) |
| Two lesions | 6 (40.00%) | |
| Three lesions | 1 (6.67%) | |
| Family history of leishmaniasis | yes | 5 (33.33%) |
| no | 10 (66.67%) | |
| Secondary infection | yes | 5 (33.33%) |
| no | 10 (66.67%) | |
| Lymph node enlargement | yes | 1 (6.67%) |
| no | 14 (93.33%) | |
| Amastigotes on histology | yes | 9 (60.00%) |
| no | 6 (40.00%) | |
| Montenegro test (mm) | Mean ± SD | 10.70 ± 1.99 |
SD = standard deviation; CI (95%) = 95% confidence interval for the mean
The mean age of the patients was 43 years, with a minimum of 16 years and maximum of 79 years, being 60% of patients male. More than half of the patients had only one lesion, and only one patient had three lesions. None of the patients had a previous history of leishmaniasis, however, five patients reported that some family member already had the disease. Secondary infection was seen before the treatment in five patients and was adequately treated with oral cephalexin before the treatment. The parasitological diagnosis was confirmed in 60% of patients with the presence of amastigotes. The remaining patients had a histology suggestive of CL. Among the changes found, the most common were chronic granulomatous dermatitis with pseudoepitheliomatous hyperplasia in the epidermis and lymphoplasmacytic inflammatory infiltrate. Montenegro test was performed in all patients, and the two-digit mean of the test result was of 10.7mm. Culture was positive for Leishmania sp in only two (25%) out of eight patients in which this exam was performed. All patients included in the study had a conventional PCR positive to Leishmania sp.
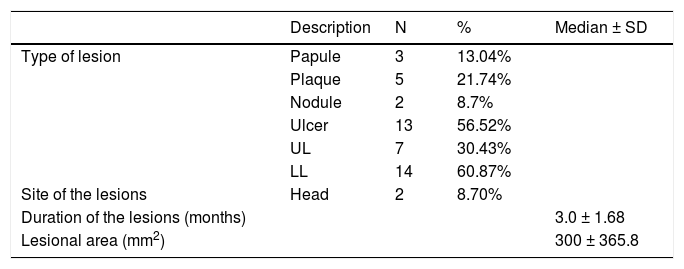
A total of 23 lesions was evaluated and of those, most were ulcers (56.52%), followed by plaques (21.74%), papules (13.04%) and nodules (8.7%). Regarding the site of the lesions, the majority was located on the lower limbs (60.87%), 30.43%, on the upper limbs and 8.7%, on the head. The mean duration of the lesions reported by the patients was around three months (Table 2).
Features of the lesions (n = 23)
| Description | N | % | Median ± SD | |
|---|---|---|---|---|
| Type of lesion | Papule | 3 | 13.04% | |
| Plaque | 5 | 21.74% | ||
| Nodule | 2 | 8.7% | ||
| Ulcer | 13 | 56.52% | ||
| UL | 7 | 30.43% | ||
| LL | 14 | 60.87% | ||
| Site of the lesions | Head | 2 | 8.70% | |
| Duration of the lesions (months) | 3.0 ± 1.68 | |||
| Lesional area (mm2) | 300 ± 365.8 |
SD = standard deviation; UL = upper limbs; LL = lower limbs.
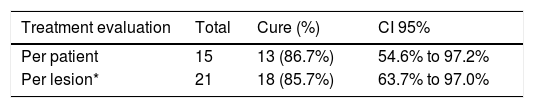
All patients returned for follow-up and there was no loss to follow-up. Complete cure in three months after the treatment was seen in 13 patients (86.67% CI 95% 54.63% to 97.23%) (Table 3). The mean cure time of the 13 patients considered to be cured was of two months. None of the patients who had complete healing of the lesions had recurrence of the lesions in 12 months of follow-up.
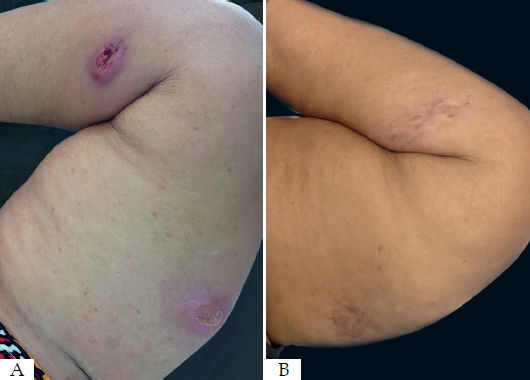
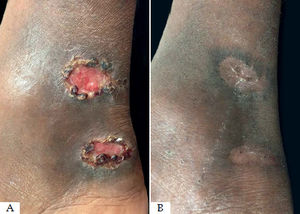
Of the seven patients with more than one lesion, only two had healing of the untreated lesions (Figure 1). Five needed radiofrequency thermotherapy for the remaining lesions and not only on the larger lesion. This happened because, after 30 days of the initial treatment, the adjacent lesions did not show signs of progression to healing and some of them even increased in size.
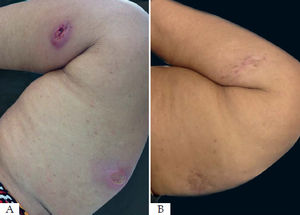
One patient did not achieve cure after three months of follow-up and refused systemic therapy with meglumine antimoniate but evolved with a good response and complete healing of her two lesions three months after another session of radiofrequency thermotherapy (Figure 2). The other patient considered as a therapeutic failure had a 75% reduction in his ulcer but did not show a complete reepithelialization of the skin to considered completely cured. This patient was treated with meglumine antimoniate.
Of the total of 23 lesions, only 15 were treated initially (one from each patient). After 30 days, the lesions were reassessed and only two (25%) of the eight untreated lesions did not progress to cure without the need of thermotherapy. Thus, of the 21 lesions treated, 18 progressed to cure after the treatment (85.7% CI 95% 63.7% to 97.0%) (Table 4). Two were excluded from the analysis because they were not treated.
Cure rate per patient and lesion after treatment with thermotherapy
| Treatment evaluation | Total | Cure (%) | CI 95% |
|---|---|---|---|
| Per patient | 15 | 13 (86.7%) | 54.6% to 97.2% |
| Per lesion* | 21 | 18 (85.7%) | 63.7% to 97.0% |
CI (95%) = 95% confidence interval for the mean.
The main side effects reported by the patients were pain (33.3%), itch (16.7%) and burning sensation (5.6%). Only one of the patients developed blisters on the application site. Seven patients (38.9%) denied any side effect. No secondary infection was seen after the treatment.
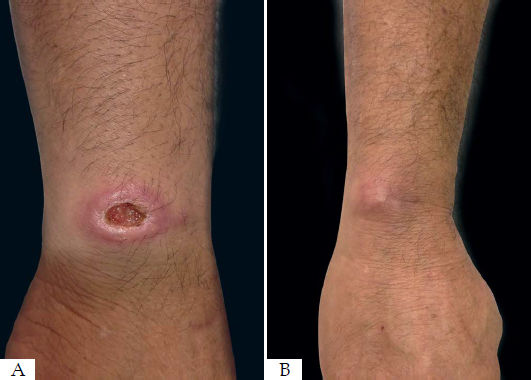
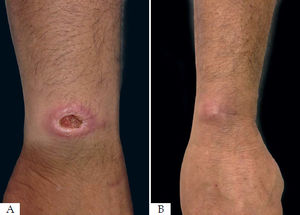
The area of reepithelialization of 13 lesions (56.5%) had an excellent aspect, acquiring almost the same color of the initial skin (Figure 3). Healing with hyperpigmentation was seen in 34.8% of the lesions, and only one patient who had two lesions healed with hypopigmentation.
DiscussionThe results of this study show that radiofrequency thermotherapy can be considered as an alternative therapy to the conventional treatment of localized CL. The sample was restricted to 15 patients because of the short interval available for data collection and the execution of the study, associated to the small number of notified cases in this period in the state of Piauí. Despite all that, we consider that this sample has demographic and clinical features that are representative of the population usually affected by this disease.
Radiofrequency thermotherapy had a cure rate of 87%, one of the highest when compared to other previous studies, even though with a broad confidence interval (CI 95% 54.6% to 97.2%). A meta-analysis of controlled clinical trials with cutaneous infection by different species evaluated the efficacy of thermotherapy and found a mean efficacy of 73.2%, inferior to the one in this study.45 The highest cure rates ever described with this treatment modality were in India (98%), in a study conducted to compare the efficacy of thermotherapy and intralesional antimonial (94%) in patients with L. tropica lesions.40 One study performed at a military hospital in Washington, with soldier that acquired the disease by L.major in Iraq or Kuwait, showed one of the lower cure rates (48%) among the studies published.39
It is possible that factors such as the host immunological response and their nutritional status, as well as differences between the species causative of the disease are responsible for the discrepancy between the cure rates found in the studies. Although in our study the Leishmania species causative of the disease was not identified, it is likely that the lesions have been caused by L. braziliensis in the majority of patients.3 A study performed previously in Guatemala with 66 patients to assess the efficacy of thermotherapy demonstrated an efficacy of 73% in patients with CL by L. braziliensis and L. mexicana, the same as patients treated with systemic pentavalent antimonial.34 In other two randomized studies that also compared thermotherapy with systemic pentavalent antimonial performed in Colombia, the cure rate was lower, around 60%. In these studies, the species responsible for the infection were L. panamensis, L. braziliensis and L. guyanensis.35,36 If we compare the results of these studies with other two involving L. tropica, we can see that the lesions caused by this species in the Old World respond more effectively to therapy with local heat, with a mean efficacy of 98% and 82.5%, respectively.40,46
Most studies demonstrated that one single application of local heat by the radiofrequency device is enough to promote a complete reepithelialization of the lesions and achieve cure. Only in two studies thermotherapy was performed in more than one session. In one of them, performed in Guatemala, the regimen was of three weekly applications of thermotherapy with a cure rate of 73%, and in the other performed in Iran, the authors opted to use the therapy once a week for four weeks, with an efficacy of 81%.34,38 A higher thermal dose with more applications or a higher temperature might improve the cure rates without increasing the side effects. However, in our study, we opted for the application of a single session of the therapy with good results.
The only study performed in Brazil with this device had the objective to determine if the healing of the CL lesions caused by therapy with local heat was associated to the modulation of the systemic response by inflammatory cytokines compared to conventional therapy with meglumine antimoniate.42 The evaluation of efficacy was impaired in this study since the patients who did not respond received the recommended treatment with pentavalent antimonial 28 days after the application of local heat. Thus, our study is groundbreaking in Brazil in regards to the evaluation of the efficacy of this alternative therapy to CL.
There is a lack of controlled studies with patients with multiple CL lesions. The aim would be to evaluate if it is necessary to treat all the lesions or if treatment of only the larger lesion with would be sufficient to induce an inflammatory response capable of promoting cure of the remaining lesions. In our study, of the seven patients with more than one lesions, only two showed healing of the untreated lesions. Five needed additional treatments for the smaller lesions, since after 30 days of follow-up they did not show any signs of reepithelialization. Of the eight lesions initially untreated, only two (25%) progressed to healing without the need of local application. This systemic effect on the untreated lesions, confirmed by this study was described in a previous report.30 The mechanism for this distance cure effect is not known. Possibly, the inflammatory response generated on the site where the heat was applied induces a systemic immune response with an effect inducing distant healing.
The patients that did not progress with proper healing three months after the treatment were considered a therapeutic failure. They had a lesion area of 1,225mm2 and 950mm2. Most patients in the study had small lesions, with a mean lesion area of 410.17mm2, being the smaller area 50mm2 and the larger 1,400mm2. It is possible that radiofrequency thermotherapy is not adequate to treat large lesions. Regardless of the treatment method, the larger the lesion, the lower the chances of cure.46 Besides, factors related to the host immune response and their nutritional status, as well as the virulence of the species involved in the disease could influence the patients’ evolution to cure.47 One of these patients had a therapeutic failure and refused to treat with systemic pentavalent antimonial. Three months after a new thermotherapy treatment, there was complete healing of the ulcers and progression to cure.
After radiofrequency thermotherapy treatment all patients were instructed to use fusidic acid cream twice daily for seven days, what probably contributed to avoid secondary infection as a complication of the treatment. Studies in which such measure was not adopted report secondary infection rates of 7% and 19%.39,41 The main side effects seen were burning sensation, pain and itch. Only one patient had a more severe reaction with blisters on the treatment site. Despite this, the patient progressed well, with cure of their ulcer and optimal healing, with mild local erythema.
All patients who responded to the treatment had an acceptable cosmetic result after 12 months of follow-up. Radiofrequency can induce collagen contraction, synthesis and remodeling.48 This way, most lesions treated acquired a color very similar to the original skin color of the patient, and the lesions that presented with depigmentation will probably improve their appearance over time, with a tendency to lightening of the hyperpigmented lesions and return of the pigmentation in hypopigmenteed lesions.
The heat produced by radiofrequency generates a local thermal dose. Thus, such as in all local therapies, it might not be able to cure distant lesions nor latent infections. This is a concern mainly in Central and South America, where CL by L. braziliensis is prevalent and the risk of the spreading of the infection to the mucous membranes continues to be worrisome. Besides, there is also the risk of indicating a local treatment for a disease that is not necessarily localized and that might have lymph node and mucosal involvement. Another disadvantage regarding thermotherapy is related to the duration of response and chances of recurrence. However, this possibility can also be extrapolated to systemic treatments that do not have enough confirmation of their efficacy in inhibiting the persistence or reactivation of parasites. In Guatemala, in more than 600 patients already treated with thermotherapy, there was no evidence of mucosal leishmaniasis after 12 months of follow-up.34 Moreover, in India, a long-term efficacy study did not show parasites in the skin through PCR even after 18 months of follow-up.40
Along the years, the therapy with pentavalent antimonial has been used as the drug of choice for the treatment of CL. However, due to its toxicity, need for laboratory tests for monitoring, difficult administration, long time for treatment, poor adherence, cost and reduced efficacy over time attributable to incomplete administration of therapeutic regimens, their use should be restricted to those situations where systemic treatment is strictly necessary. Among these situations are cases of disseminated mucosal leishmaniasis, diffuse leishmaniasis and sites where heat therapy should be avoided, such as areas close to mucous membranes. Thus, most patients with few lesions of localized CL would be spared from a treatment more aggressive than the disease itself. This benefit would be especially important for elderly patients. Many times, this group of patients receive a toxic systemic treatment because of an ulcerated lesion that was not even confirmed to be CL with the parasitological methods. This would avoid fatal outcomes such as the ones previously reported.17,18
One of the main ways found to lessen the disadvantages of the use of systemic pentavalent antimonial is using it intralesionally. With this, most of the side effects are minimized. The efficacy of intralesional antimonial was already registered and it ranges from 80-92%.40,49 However, studies published in Iran show a not so high cure rate for this treatment modality, with an efficacy between 40-60%.38,50 Other disadvantages include the need for multiple applications, reducing patient adherence, a larger amount of side effects when compared to thermotherapy, the cost and the need for laboratory monitoring.
New therapeutic options have been sought. However, despite good results, none of them was considered convenient or sufficiently effective to replace conventional treatment. Among the localized treatment options, radiofrequency thermotherapy appears as a viable option, since it is effective and requires only one session, improving patient adherence. One of the main advantages of this therapy is that the device is easy to use, light and works with rechargeable batteries, making it possible to be used in rural areas or areas with no electricity. Another advantage of this treatment modality is that it does not require monitoring with laboratory tests, saving additional costs for health institutions. Although the thermotherapy device is still relatively expensive, making it of difficult access in endemic countries, the costs related to conventional treatment are higher. Another possibility to be investigated is the association of thermotherapy to new oral treatment options, such as miltefosine, for example. All these advantages make this therapy a good alternative for the treatment of localized CL, particularly in patients who have absolute or relative contraindications to the conventional therapy, such as heart, kidney and liver conditions, pregnant women and HIV-positive patients.
ConclusionsTaking the results of this study into consideration, we conclude that radiofrequency thermotherapy can be considered as a possible alternative to pentavalent antimonials for the treatment of localized CL in Brazil, especially in cases of a single lesion and when there is formal contraindication to conventional treatment.
Additional randomized controlled studies are needed and are being conducted with a larger number of patients and a longer follow-up period, so that this treatment modality be considered as one of the main alternatives to conventional treatment for localized CL in Brazil.
Financial support: FADEX - Fundação Cultural e de Fomento à Pesquisa, Ensino e Extensão e Inovação.
Conflict of interest: None.