Female pattern hair loss (FPHL) is a common complaint in adult women and inflicts major impact in quality of life, however, there is no specific questionnaire available in Portuguese for such evaluation.
Objectives:Translation into Brazilian Portuguese, cultural adaptation and validation of the WAA-QoL (Women’s Androgenetic Alopecia Quality of Life Questionnaire).
Methods: Methodological study. After authorization by the author, cultural (linguistic) translation and adaptation to Portuguese of the WAA-QoL questionnaire were carried out. The translated version (WAA-QoL-BP) and DLQI (Dermatology Life Quality index) were submitted to patients with FPHL for concurrent validation. Twenty patients were reevaluated to assess temporal stability.
Results:A total of 116 patients with APF were evaluated, the mean age (SD) was 47 (14) years, and 89 (76%) patients were classified as grades II and III (Sinclair). There was high internal consistency: Cronbach’s alpha was 0.97 for the WAA-QoL-BP and 0.87 for the DLQI. The correlation between WAA-QoL and DLQI resulted in (rho) 0.81 (p <0.01). The intraclass correlation coefficient for complete agreement of WAA-QoL-BP was 0.95 (p <0.01) in the test-retest comparison.
Study limitations:Sampling of patients only from the State of São Paulo.
Conclusions:A Brazilian version for WAA-QoL was translated and adapted, which proved to be valid and consistent.
Female pattern hair loss (FPHL) is the main cause of hair loss in adult women. Its prevalence is estimated in around 25% at the age of 50 years, with proportional increase to age, mainly among Caucasians. It is characterized by progressive follicular miniaturization of unclear etiology, but it is influenced by multiple factors such as genetic predisposition, hormonal and environmental changes, being sun exposure and smoking among them.1-3
FPHL represents significant demand to dermatological care and, despite being asymptomatic, causes a great impact on the quality of life (QoL).4-8 In a national survey with healthy population, the fear of losing hair was similar to that of having a heart attack.9
The World Health Organization defines QoL as “the perception the individual has of their position in life, the context of the culture and value systems in which they live in, in relation to their goals, expectations, standards and concerns”.10
The WAA-QoL (Women’s Androgenetic Alopecia Quality of Life Questionnaire) is the only available specific instrument to evaluate QoL in FPHL patients. It is made of 16 questions with 6 alternatives, self-administered, and has not yet been adapted to Brazil.11
In this study, we aimed at translating into Brazilian Portuguese, culturally adapting, and validating WAA-QoL. Secondarily, we evaluated factors associated to QoL in FPHL.
MethodsMethodological study, carried out after authorization of the authors-copyright owners of WAA-QoL, who detain the reproduction rights of the instrument (Tara Robbins; Merck Inc.).11 The project was approved by the Ethics Committee of the institution (CAAE: 58124816.7.0000.5515).
The questionnaire was translated by three fluent dermatologists who created a consensual version, submitted to 10 FPHL patients for cultural adaptation. The final version was translated back into English by a native speaker and presented to the authors for approval.12
The WAA-QoL-BP was applied to a group of 116 women with FPHL, from both a public university service (Unesp – Botucatu - SP and Unoeste - Presidente Prudente - SP) and private practices of the researchers and clinical and epidemiological aspects and the questionnaire DLQI-BRA (Dermatology Life Quality Index) were also evaluated, in order to provide concurrent validation.13,14 The questionnaires were self-administered by the participants and applied by the authors.
A subgroup of 20 was reassessed after 1 to 4 weeks to estimate the temporal stability of the construct.
The diagnosis of FPHL was based in the clinical picture and the presence of more than 20% of miniaturized hairs on dermoscopy.1 The study was conducted between May/2016 and May/2017.
Data normality was evaluated by the Shapiro-Wilk test. The overall internal consistency of the instruments was evaluated by Cronbach’s alpha. The correlation between the questionnaires and between the items was tested by Spearman’s rho. The temporal stability was estimated by the intraclass correlation coefficient for complete concordance and laid out in the Bland-Altman plot. WAA-QoL’s dimensionality were tested by Horn’s (parallel analysis) and Hull’s method, and their scores were tested regarding the influence of clinical-epidemiological variables by a generalized linear model.15-19
The informativeness obtained from the items in the WAA-QoL-BP was assessed from a response model to the polytomic item for ordinal data (Samejima graded model).20
Data were analyzed by the software IBM SPSS 22, eIRT 1.3.0 and FACTOR 10.5.21,22 A p<0.05 was considered significant.16
The sample was calculated based on a pre-test with the first 50 interviews, in order to satisfy a generalized linear model with 11 co-variables, defining alpha error of 5% and beta of 20%.16,23
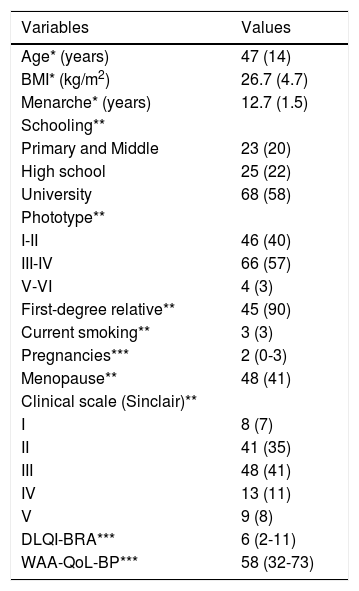
ResultsThe main clinical, epidemiological and QoL data of the 116 women evaluated are shown in table 1. We highlight adult age (>30 years), a high frequency of family history and a moderate impact in the quality of life caused by the dermatological condition. All participants were literate.
Main clinical, epidemiological and quality of life data of the patients assessed (n=116)
| Variables | Values |
|---|---|
| Age* (years) | 47 (14) |
| BMI* (kg/m2) | 26.7 (4.7) |
| Menarche* (years) | 12.7 (1.5) |
| Schooling** | |
| Primary and Middle | 23 (20) |
| High school | 25 (22) |
| University | 68 (58) |
| Phototype** | |
| I-II | 46 (40) |
| III-IV | 66 (57) |
| V-VI | 4 (3) |
| First-degree relative** | 45 (90) |
| Current smoking** | 3 (3) |
| Pregnancies*** | 2 (0-3) |
| Menopause** | 48 (41) |
| Clinical scale (Sinclair)** | |
| I | 8 (7) |
| II | 41 (35) |
| III | 48 (41) |
| IV | 13 (11) |
| V | 9 (8) |
| DLQI-BRA*** | 6 (2-11) |
| WAA-QoL-BP*** | 58 (32-73) |
The distribution of the scores of each WAA-QoL-BP item is represented in figure 1. The items 7, 8, 11, 12 and 13 stand out as those of highest impact. Only items 5 and 6 (socialization and relationship with the opposite sex) expressed floor effect, with around 40-50% of the scores at level zero (nothing). The time to fill out the questionnaire was of less than 10 minutes for all patients.
The internal consistency of WAA-QoL-BP resulted 0.97 and of DLQI-BRA, 0.87. Both Horn’s parallel analysis and Hull method revealed unidimensionality for WAA-QoL-BP.
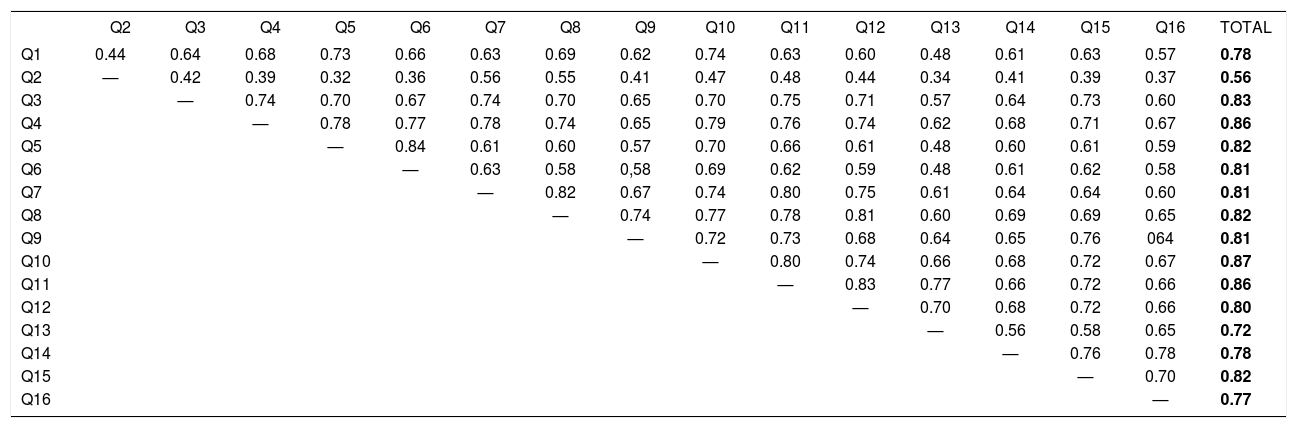
The correlation between the total scores of the questionnaires WAA-QoL-BP and DLQI-BRA was of 0.81 (p<0.01). the item-item and item-total correlation for WAA-QoL-BP are shown in table 2. Of note, most correlations are above 0.60.
Spearman’s correlation coefficient (rho) between the items and the total score of the WAA-QoL-BP (n=116)
| Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | TOTAL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | 0.44 | 0.64 | 0.68 | 0.73 | 0.66 | 0.63 | 0.69 | 0.62 | 0.74 | 0.63 | 0.60 | 0.48 | 0.61 | 0.63 | 0.57 | 0.78 |
| Q2 | — | 0.42 | 0.39 | 0.32 | 0.36 | 0.56 | 0.55 | 0.41 | 0.47 | 0.48 | 0.44 | 0.34 | 0.41 | 0.39 | 0.37 | 0.56 |
| Q3 | — | 0.74 | 0.70 | 0.67 | 0.74 | 0.70 | 0.65 | 0.70 | 0.75 | 0.71 | 0.57 | 0.64 | 0.73 | 0.60 | 0.83 | |
| Q4 | — | 0.78 | 0.77 | 0.78 | 0.74 | 0.65 | 0.79 | 0.76 | 0.74 | 0.62 | 0.68 | 0.71 | 0.67 | 0.86 | ||
| Q5 | — | 0.84 | 0.61 | 0.60 | 0.57 | 0.70 | 0.66 | 0.61 | 0.48 | 0.60 | 0.61 | 0.59 | 0.82 | |||
| Q6 | — | 0.63 | 0.58 | 0,58 | 0.69 | 0.62 | 0.59 | 0.48 | 0.61 | 0.62 | 0.58 | 0.81 | ||||
| Q7 | — | 0.82 | 0.67 | 0.74 | 0.80 | 0.75 | 0.61 | 0.64 | 0.64 | 0.60 | 0.81 | |||||
| Q8 | — | 0.74 | 0.77 | 0.78 | 0.81 | 0.60 | 0.69 | 0.69 | 0.65 | 0.82 | ||||||
| Q9 | — | 0.72 | 0.73 | 0.68 | 0.64 | 0.65 | 0.76 | 064 | 0.81 | |||||||
| Q10 | — | 0.80 | 0.74 | 0.66 | 0.68 | 0.72 | 0.67 | 0.87 | ||||||||
| Q11 | — | 0.83 | 0.77 | 0.66 | 0.72 | 0.66 | 0.86 | |||||||||
| Q12 | — | 0.70 | 0.68 | 0.72 | 0.66 | 0.80 | ||||||||||
| Q13 | — | 0.56 | 0.58 | 0.65 | 0.72 | |||||||||||
| Q14 | — | 0.76 | 0.78 | 0.78 | ||||||||||||
| Q15 | — | 0.70 | 0.82 | |||||||||||||
| Q16 | — | 0.77 |
All correlations resulted if p<0.01.
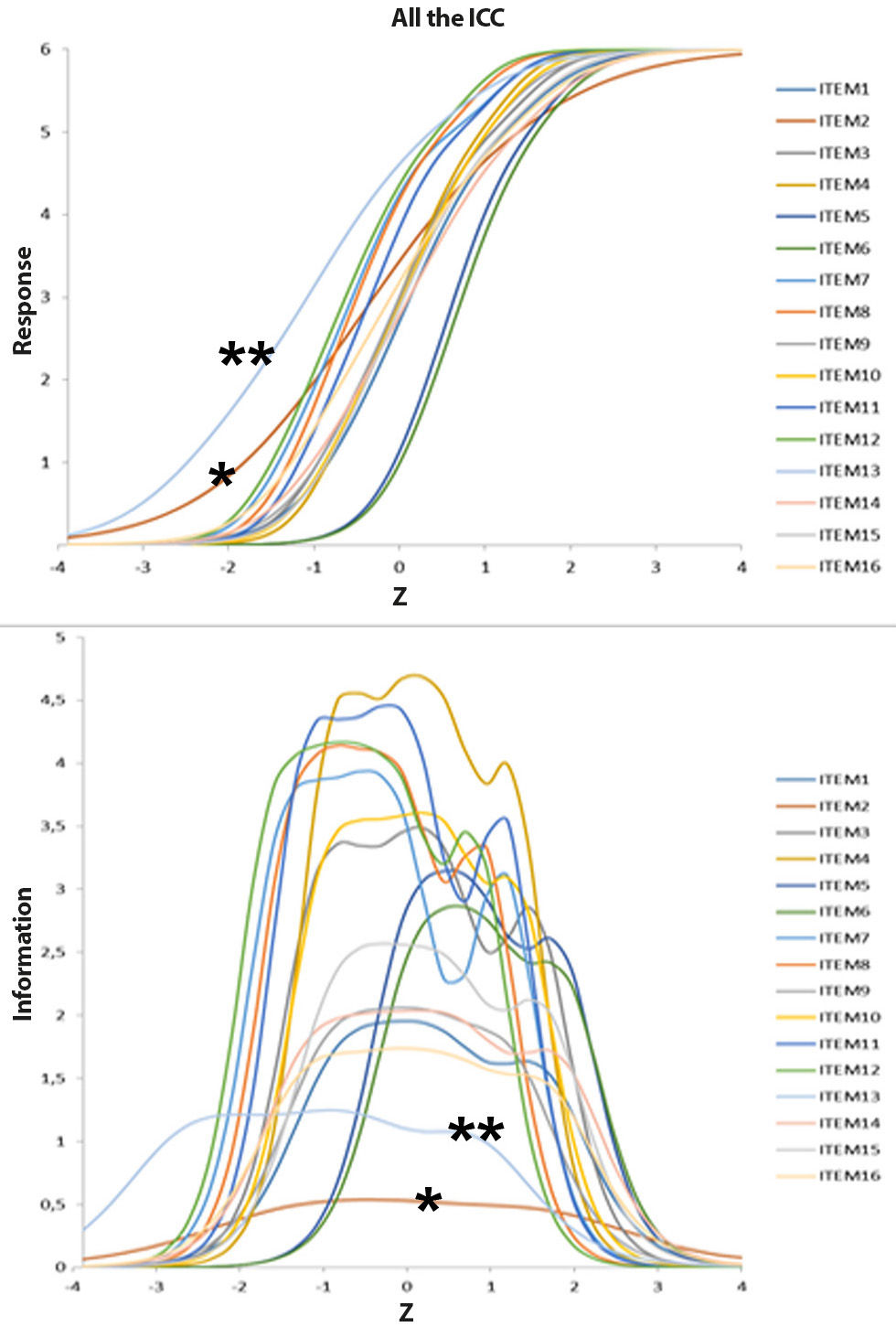
According to the function analysis of information based in the theory of response to the item, all items showed an adequate convergence to the model (p>0.95). The typical item curves and information functions are laid out in figure 2. Items 2 and 13 stand out with smaller discriminations and information depending on the sample.
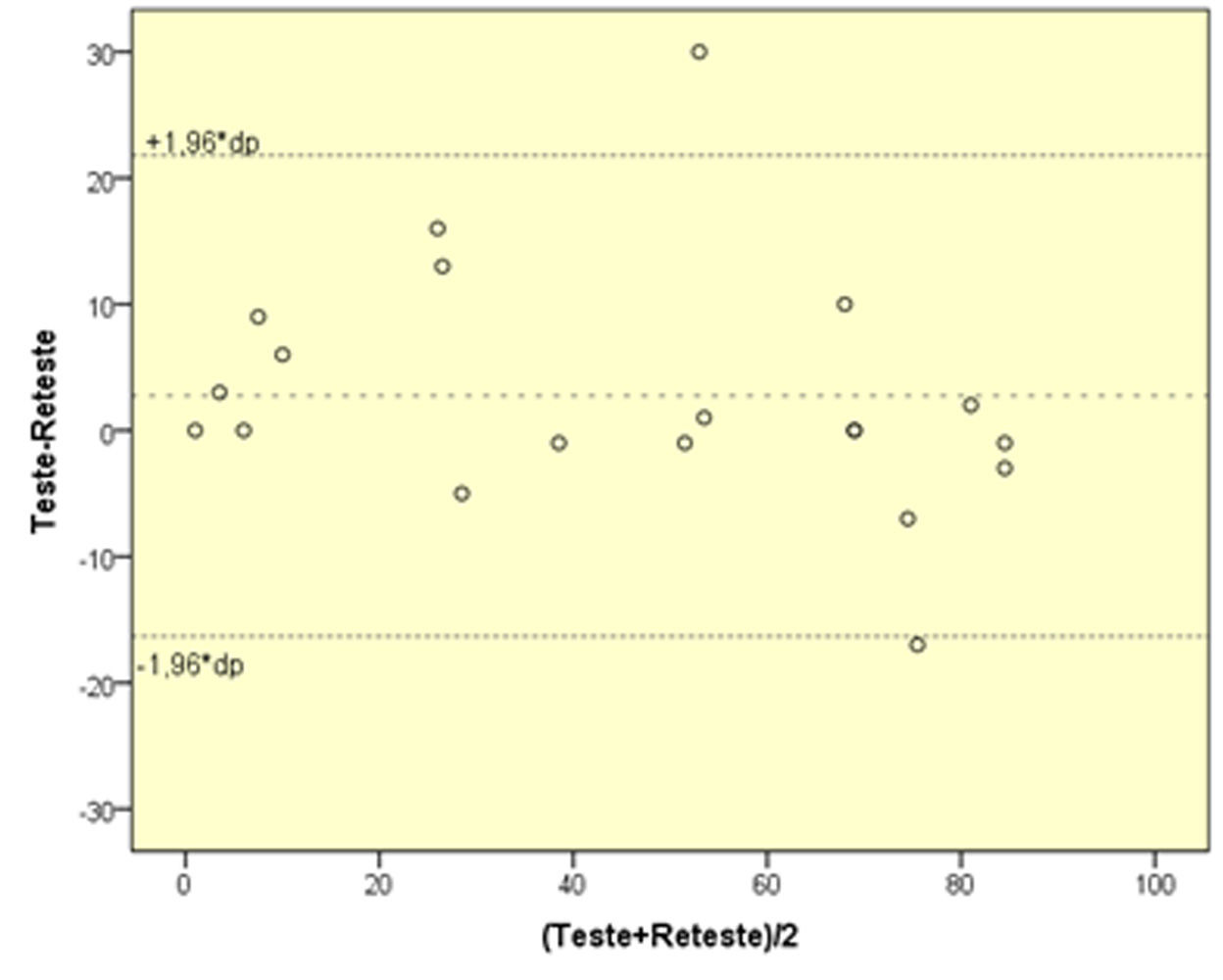
Twenty patients were retested between 1-4 weeks (Figure 3). The intraclass correlation coefficient for complete concordance was 0.95 (p<0.01).
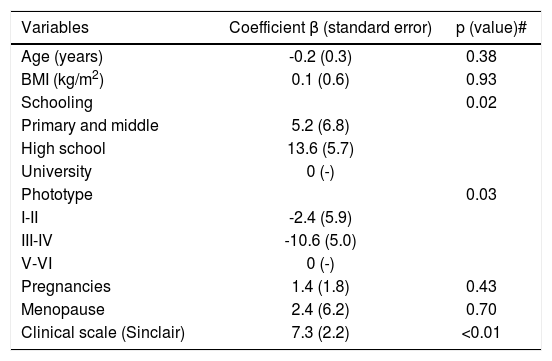
The factors associated to higher scores of WAA-QoL are shown in table 3. Higher scores in more severe cases, poorer schooling and higher phototypes stand out.
Generalized linear model of the clinical and demographical elements related to WAA-QoL-BP scores (n=116)
| Variables | Coefficient β (standard error) | p (value)# |
|---|---|---|
| Age (years) | -0.2 (0.3) | 0.38 |
| BMI (kg/m2) | 0.1 (0.6) | 0.93 |
| Schooling | 0.02 | |
| Primary and middle | 5.2 (6.8) | |
| High school | 13.6 (5.7) | |
| University | 0 (-) | |
| Phototype | 0.03 | |
| I-II | -2.4 (5.9) | |
| III-IV | -10.6 (5.0) | |
| V-VI | 0 (-) | |
| Pregnancies | 1.4 (1.8) | 0.43 |
| Menopause | 2.4 (6.2) | 0.70 |
| Clinical scale (Sinclair) | 7.3 (2.2) | <0.01 |
p (model)=0.01; p (interception)<0.01; p (valor) # multivariate.
The symbolic representation of hair is very important for females, and alopecia represents a great concern, impacting on the quality of life, attractiveness and self-esteem.7,8 A Brazilian study showed that the fear of losing all hair is similar to that of developing cancer or having a heart attack.9
Specific questionnaires for the evaluation of QoL are preferred to generic questionnaires because they were developed based on the areas that are specifically affected by the disease being studied, delivering more precise results. In fact, the internal consistency of the WAA-QoL responses was higher than the DLQI.
WAA-QoL was developed from the reduction of 185 items from the focal group and the literature, evaluated by 120 women with FPHL.11 The final questionnaire showed excellent internal consistency and temporal stability, similar to those found in our study. In this study, the Brazilian version (WAA-QoL-BP) showed an adequate psychometric performance, informativeness, feasibility and temporal stability, indicating its use as outcome in clinical studies.
FPHL has a chronic course and slow response to treatment. The score variation of WAA-QoL-BP can be explained not only by the severity of the condition, but also indicates other perceptive elements involved. The items with higher scores in the WAA-QoL-BP were related to the appearance of hair, possibility of styling/combing the hair, to the frustration with the condition and to the fear of the persistence of hair loss.
The understanding of which elements impact the QoL of patients with FPHL favors the identification of individual actions that result in the reduction of the suffering caused by the condition such as scalp covers, partial prosthesis, besides realistic perspectives regarding treatment. These results indicate the need of future studies that explore interventions besides specific treatment for FPHL.
The limitation of this study is that it is monocentric, despite the adequate sample in relation to schooling levels, phototypes and age groups.
ConclusionA Brazilian version of the WAA-QoL was translated and adapted, which proved to be valid and consistent.
Financial support: None.
Conflict of interest: None.