There is little data in the literature concerning dermatologic admissions. Several diseases are seasonal in incidence and clinical worsening. We performed a survey of hospitalizations in the dermatology ward of a public hospital (April/2007 to May/2017). There were 1790 hospitalizations, whose main diagnoses were infectious dermatoses, neoplasias, psoriasis, bullous diseases and cutaneous ulcers. In winter, there were fewer hospitalizations for bacterial infections and urticaria, but more for leprosy. In summer, there were fewer hospitalizations for systemic and subcutaneous mycoses, but more for zoodermatoses and erythema multiforme. In the fall, more patients were admitted with mycoses. Spring favored urticaria and angioedema, but less cases of erythema multiforme and diabetic foot.
Dermatologists practice predominantly in the outpatient setting but there are severe skin conditions that represent life risks or require admission for specialized treatment. However, there is limited data in the literature about skin conditions that might require hospital admission.1,2
As with some conditions (for example, asthma, influenza and depression), many skin disorders are seasonal in regards to their incidence or clinical worsening, such as actinic keratoses and melasma in summer, psoriasis and atopic dermatitis in winter, pityriasis rosea in spring, among others.3–5
Seasonality interferes in the course and efficacy of the treatment of certain conditions due to the pathophysiological basis of the disease, climatic temperature regimens and humidity or exposure to environmental triggers related to the astronomic season.6
Since hospital admissions are indicators of the severity of the dermatological clinical picture, investigation of seasonality of admissions reveals the profile of exacerbation or incidence, what generates relevant epidemiological data in collective health. The objective of this study was to explore the seasonality of groups of skin conditions that required admission into a public hospital in Brazil.
We conducted a retrospective study that analyzed data related to the admission of patients based on the registry of a dermatology ward of a public university service (Unesp, Botucatu, SP, Brazil; latitude: 22°53°09″S; longitude: 48°26°42″W; height: 804 m) between the months of April 2007 and May 2017.
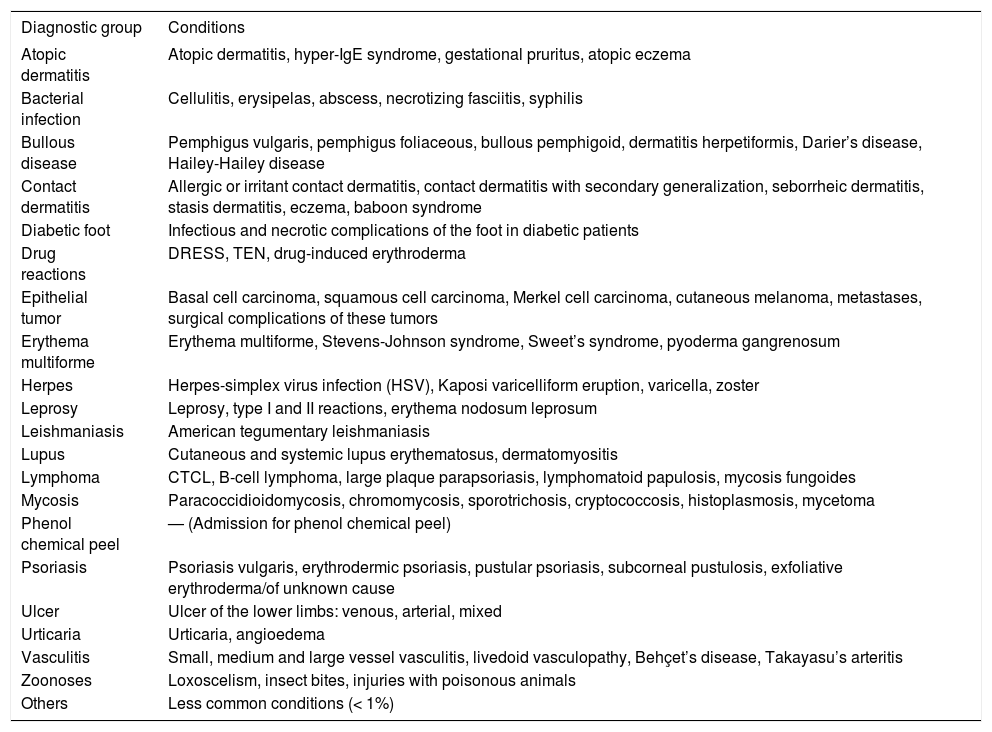
Demographical and clinical characteristics were evaluated, as well as those related to the time of admission. The diagnoses were grouped into 21 categories (Table 1).
Categories of dermatological diagnoses grouped by similarity
| Diagnostic group | Conditions |
|---|---|
| Atopic dermatitis | Atopic dermatitis, hyper-IgE syndrome, gestational pruritus, atopic eczema |
| Bacterial infection | Cellulitis, erysipelas, abscess, necrotizing fasciitis, syphilis |
| Bullous disease | Pemphigus vulgaris, pemphigus foliaceous, bullous pemphigoid, dermatitis herpetiformis, Darier’s disease, Hailey-Hailey disease |
| Contact dermatitis | Allergic or irritant contact dermatitis, contact dermatitis with secondary generalization, seborrheic dermatitis, stasis dermatitis, eczema, baboon syndrome |
| Diabetic foot | Infectious and necrotic complications of the foot in diabetic patients |
| Drug reactions | DRESS, TEN, drug-induced erythroderma |
| Epithelial tumor | Basal cell carcinoma, squamous cell carcinoma, Merkel cell carcinoma, cutaneous melanoma, metastases, surgical complications of these tumors |
| Erythema multiforme | Erythema multiforme, Stevens-Johnson syndrome, Sweet’s syndrome, pyoderma gangrenosum |
| Herpes | Herpes-simplex virus infection (HSV), Kaposi varicelliform eruption, varicella, zoster |
| Leprosy | Leprosy, type I and II reactions, erythema nodosum leprosum |
| Leishmaniasis | American tegumentary leishmaniasis |
| Lupus | Cutaneous and systemic lupus erythematosus, dermatomyositis |
| Lymphoma | CTCL, B-cell lymphoma, large plaque parapsoriasis, lymphomatoid papulosis, mycosis fungoides |
| Mycosis | Paracoccidioidomycosis, chromomycosis, sporotrichosis, cryptococcosis, histoplasmosis, mycetoma |
| Phenol chemical peel | — (Admission for phenol chemical peel) |
| Psoriasis | Psoriasis vulgaris, erythrodermic psoriasis, pustular psoriasis, subcorneal pustulosis, exfoliative erythroderma/of unknown cause |
| Ulcer | Ulcer of the lower limbs: venous, arterial, mixed |
| Urticaria | Urticaria, angioedema |
| Vasculitis | Small, medium and large vessel vasculitis, livedoid vasculopathy, Behçet’s disease, Takayasu’s arteritis |
| Zoonoses | Loxoscelism, insect bites, injuries with poisonous animals |
| Others | Less common conditions (< 1%) |
Categorical variables were represented by percentage and compared by Chi-square and by the analysis of residues (standardized and adjusted) of the contingency table. Quantitative variables were represented by means and standard deviations or medians and quartiles (p25–p75) and compared with the Student’s T and Mann-Whitney tests, if indicated. We considered significant a value of p ≤ 0.05.
In total, 1790 dermatological admissions were evaluated in a period of 10.1 years, with 941 (53%) male patients. The mean age (sd) was 55 (19) years. The median (p25–p75) of the length of admissions was 9 (5–15) days. Men were older on admission [57 (19) years vs. 54 (20) years (p < 0.01)] and had longer length of hospital stay [10 (6–16) days vs. 8 (5–14) days (p < 0.01)].
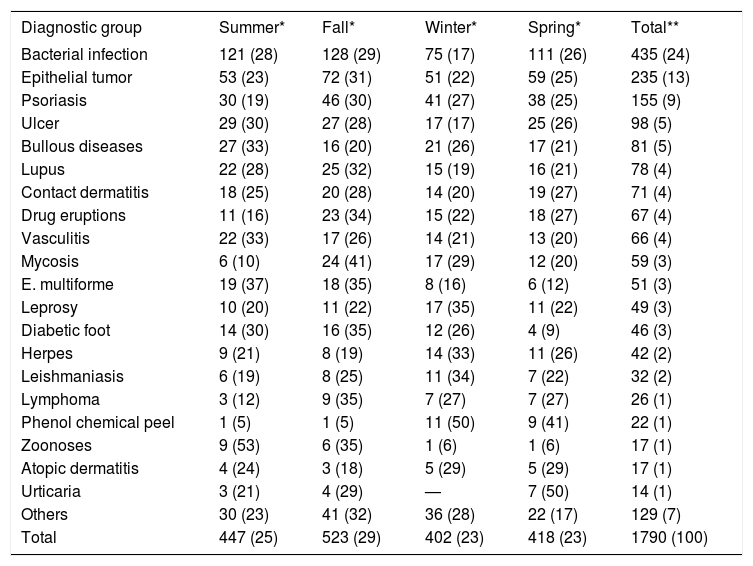
Diagnoses were grouped into categories and evaluated according to seasonality of admissions (Table 2). The main reasons for admission (56%) were infectious conditions, epithelial neoplasms and psoriasis.
Seasonal incidence of the diagnostic groups. In blue, the diagnoses that exceeded the expected frequencies for the season (p ≤ 0.05); in red, those that were less frequent (p ≤ 0.05)
| Diagnostic group | Summer* | Fall* | Winter* | Spring* | Total** |
|---|---|---|---|---|---|
| Bacterial infection | 121 (28) | 128 (29) | 75 (17) | 111 (26) | 435 (24) |
| Epithelial tumor | 53 (23) | 72 (31) | 51 (22) | 59 (25) | 235 (13) |
| Psoriasis | 30 (19) | 46 (30) | 41 (27) | 38 (25) | 155 (9) |
| Ulcer | 29 (30) | 27 (28) | 17 (17) | 25 (26) | 98 (5) |
| Bullous diseases | 27 (33) | 16 (20) | 21 (26) | 17 (21) | 81 (5) |
| Lupus | 22 (28) | 25 (32) | 15 (19) | 16 (21) | 78 (4) |
| Contact dermatitis | 18 (25) | 20 (28) | 14 (20) | 19 (27) | 71 (4) |
| Drug eruptions | 11 (16) | 23 (34) | 15 (22) | 18 (27) | 67 (4) |
| Vasculitis | 22 (33) | 17 (26) | 14 (21) | 13 (20) | 66 (4) |
| Mycosis | 6 (10) | 24 (41) | 17 (29) | 12 (20) | 59 (3) |
| E. multiforme | 19 (37) | 18 (35) | 8 (16) | 6 (12) | 51 (3) |
| Leprosy | 10 (20) | 11 (22) | 17 (35) | 11 (22) | 49 (3) |
| Diabetic foot | 14 (30) | 16 (35) | 12 (26) | 4 (9) | 46 (3) |
| Herpes | 9 (21) | 8 (19) | 14 (33) | 11 (26) | 42 (2) |
| Leishmaniasis | 6 (19) | 8 (25) | 11 (34) | 7 (22) | 32 (2) |
| Lymphoma | 3 (12) | 9 (35) | 7 (27) | 7 (27) | 26 (1) |
| Phenol chemical peel | 1 (5) | 1 (5) | 11 (50) | 9 (41) | 22 (1) |
| Zoonoses | 9 (53) | 6 (35) | 1 (6) | 1 (6) | 17 (1) |
| Atopic dermatitis | 4 (24) | 3 (18) | 5 (29) | 5 (29) | 17 (1) |
| Urticaria | 3 (21) | 4 (29) | — | 7 (50) | 14 (1) |
| Others | 30 (23) | 41 (32) | 36 (28) | 22 (17) | 129 (7) |
| Total | 447 (25) | 523 (29) | 402 (23) | 418 (23) | 1790 (100) |
Chi square = 104; 60 gl; p < 0.01
Blue: p ≤ 0.05; higher than expect; red: p ≤ 0.05; lower than expected.
Stays longer than 10 days were due to leprosy, leishmaniasis, ulcers, mycoses, diabetic foot, psoriasis, zoonoses and bullous diseases; stays shorter than 8 days were due to phenol peels, urticaria, lupus, vasculitis, contact dermatitis, erythema multiforme and tumors.
Of note, in winter there was a lower incidence of bacterial infections but a higher incidence of leprosy and admissions for deep chemical peels. In summer months, there was a lower incidence of deep mycoses but a higher incidence of zoonoses and erythema multiforme. Spring favored the incidence of urticaria and angioedema, with less frequent admissions for diabetic foot and erythema multiforme. In fall, more patients with mycoses were admitted.
Although not statistically significant (p = 0.09), there were less admissions for psoriasis and drug reactions in summer.
Twenty-six (1.5%) deaths were seen during admission. We highlight as causes: infectious diseases, neoplasms and bullous diseases, besides advanced age (older than 70 years).
This study identified groups of skin conditions that lead to admission associated to the seasons. The subtropical climate of the region studied, with well-defined seasons regarding temperature, sun exposure and rain, favors the investigation of which climatic, exposure or behavioral factors interfere in the incidence and severity of cutaneous diseases.
Hospital stay, prevalence of diagnosis and mortality patterns are similar to other case series.1,2
The skin, as a contact organ, is susceptible to the effect of environmental triggers for the development of diseases. Ultraviolet radiation has a therapeutic effect in psoriasis and pityriasis rosea, but on the other hand is aggravating for photodermatoses such as drug-induced photosensitivity, lupus and polymorphic eruption. Our studies suggest a less marked worsening of psoriasis in summer.
With the heat, there is increased activity of insects and poisonous animals and a higher exposure to outdoor activities, such as fishing and walks, what could explain the frequency of zoonoses at this time of the year.6,7
Some authors identified higher incidences of staphylococcal infections in the hotter and more humid months, explaining lower admission rates for bacterial infections in winter, which is dry and cold in the region.8 In the same way, the winter climate favors respiratory infections, which are associated to immune imbalances that can trigger leprosy reactions.9,10 Urticaria and rhinitis are associated to airborne allergens, more widespread in spring.
Besides drawing attention to an epidemiological alert for the control and prevention of damage, this study values the hospital integration of the dermatologist and directs the need for ongoing updates on these conditions, particularly with the progressive aging of the population.
This study presents limitations related to the accuracy of clinical records, limitations of the sensitivity of the method for identification of patterns in rare diseases and limitations since a large fraction of the diagnoses identified are treated in an outpatient setting.
The generalization of the results is impaired by the reality of the sample population: public service in a subtropical region. However, the discrepancies observed indicate biological or exposure elements that can be observed in patients from other regions or subject to travelling. Similar studies in different geopolitical conditions should investigate the seasonality of dermatological admissions according to each context.
Development and urbanization promote, besides increased life expectancy, an epidemiological transition altering the prevalences of diseases; however, that should not significantly interfere in their seasonality.
Many skin conditions that require hospital admission have seasonal patterns that should be taken into consideration for sizing the service and for primary prevention in the population.
Financial support: None.
Conflict of interest: None.