Solar urticaria is a rare form of physical urticaria mediated by immunoglobulin E. The lesions appear immediately after the sun exposure, interfering with the patient’s normal daily life. Omalizumab, a monoclonal anti-IgE antibody, has been recently approved for the treatment of chronic spontaneous urticaria, and the latest reports support its role also in the treatment of solar urticaria. Hereby, we report a case of solar urticaria refractory to conventional treatment strategies, with an excellent response to treatment with omalizumab and phototesting normalization.
Solar urticaria (SU) is a rare photodermatosis characterized by rapid induction of symptoms and onset of skin lesions after exposure to sunlight. The pathogenesis of the disease has yet not been fully elucidated. Currently, the onset of symptoms is considered to be triggered by a yet to be identified chromophore, which is activated by light of a particular wavelength.1 The diagnostic process is based on the characteristic clinical history, completed by phototesting to determine the specific wavelength, the triggering spectrum of the light, responsible for the symptoms. Visible light and UVA rays are the most frequently involved.2 Solar urticaria has a significant impact on the quality of life of patients, therefore requiring an effective therapy. However, the treatment is challenging and the combination of several therapeutic modalities is almost always necessary.1,3 Recently, some cases of US with successful treatment with omalizumab have been published.4
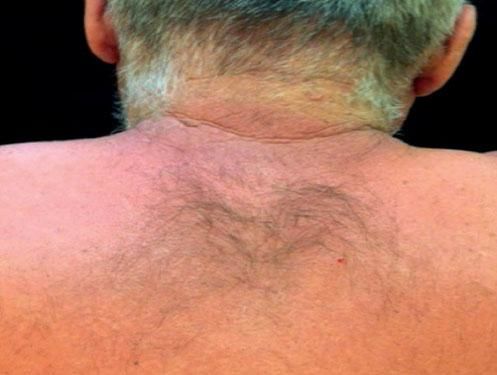
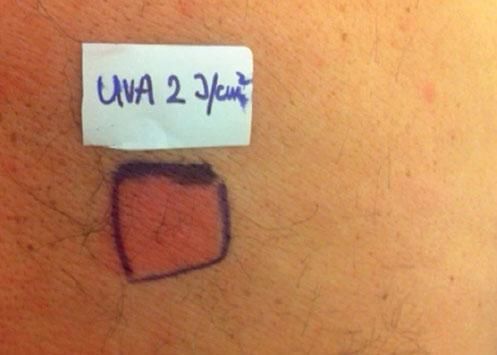
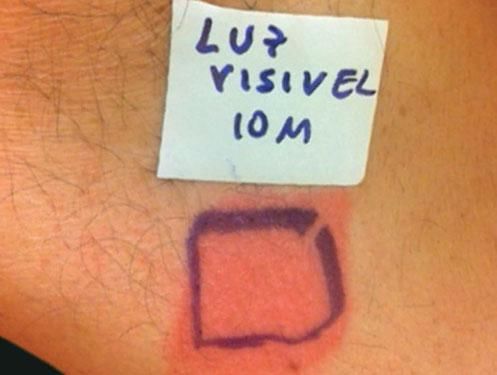
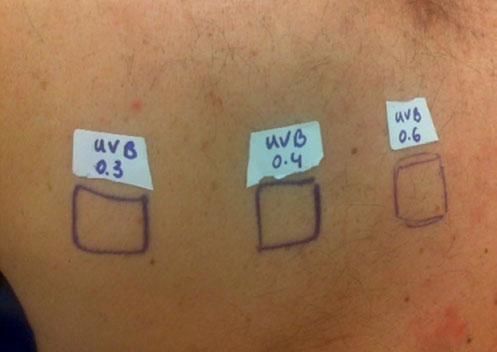
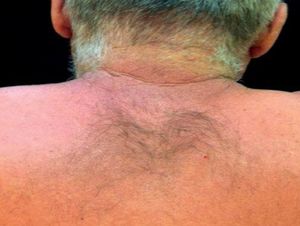
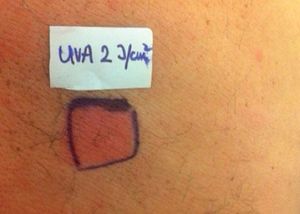
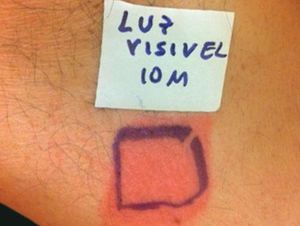
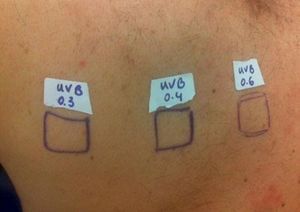
Case ReportThe authors report a case of a 60-year-old man with a medical history of familiar hypercholesterolemia and solitary kidney. His medication included trazodone, rosuvastatin and fenofibrate. The patient presented to the dermatology department with a 2-year history of an itchy skin eruption. The lesions consisted of short-lasting erythematous patches and wheals, with onset within 5 to 10 minutes after exposure to direct sunlight, appearing initially on the sun-exposed areas, and later on covered ones (Figure 1). The lesions occurred daily, immediately after exposure to daylight, leading to a significant decrease in the quality of life. The patient experienced lipothymia and generalized erythema after short sun exposure on the beach, resulting in an urgent visit to the emergency department and a consequent complete daylight avoidance. Laboratory studies were normal and to evaluate the involved spectrum of light triggering the lesions, phototesting was performed in the phototherapy room. Exposure to the light from the projector showed reaction after 10 minutes, just as the exposure to UVA light (Waldmanno 7001K), with positive reaction with 2J/cm2 (Figures 2 and 3). UVB phototesting was negative (Figure 4). Phototesting confirmed the diagnosis of SU to visible light and to UVA.
In this patient, besides the adoption of photoprotection measures, diverse conventional therapies for SU were tried: H1 second generation antihistamines, hydroxychloroquine, azathioprine and low-dose prednisolone. However, the response was poor or incomplete and the patient maintained symptoms. Phototherapy was not performed due to logistic issues. Hypolipidemic drugs were suspended during two months, with no improvement. Omalizumab was commenced at the dose of 300mg s.c. and administered at monthly intervals. The patient achieved clinical remission during the first month of treatment and is still asymptomatic. After 6 months of treatment, we repeated phototesting, which turned out to be negative. No adverse reaction was registered during 16 months of treatment.
DiscussionSolar urticaria belongs to the group of chronic inducible urticarias, with a severe impact in the patient’s daily life. As the presented case shows, there might be a risk of generalized reactions with anaphylaxis upon exposure of large body surface area. The disease course is chronic, with only few cases of complete resolution.5 With all this in mind, it is crucial to achieve the control of the disease. The treatment of SU can be very challenging. Sun protective measures to minimize the exposure should be adopted by all patients. The conventional treatment usually leads only to a modest clinical improvement and consists of high dose of second generation H1 antihistamines in monotherapy or in combination with immunosuppressive drugs, phototherapy or photochemotherapy for desensitization, plasmapheresis and intravenous immunoglobulin.1,3 In the presented case, several therapeutic options were combined, still with insufficient control of urticaria.
Taking into account the well-known efficacy of omalizumab in the treatment of chronic spontaneous urticaria (CSU), there have been several cases recently reported of its efficacy also in patients with SU. To date, 20 articles have been published, the majority being case reports or small case series and one multicentric prospective study in France.6-8
Forty-five cases of SU treated with omalizumab (including the patient presented in this case report) were identified. In 36 patients (80%), the symptoms were controlled successfully. In 9 cases (20%), the treatment response was partial or insufficient. The treatment schemes differ from patient to patient, with maintenance dosage 150-450 mg every 2 weeks or monthly.4 In the presented case, we adopted the dosing as indicated for CSU, with an excellent response. Further studies are warranted to establish the correct therapeutic scheme, but certainly omalizumab will remain a valid and useful option, due to its efficacy and safety profile presented so far.