The low prevalence of erythromelalgia, classified as an orphan disease, poses diagnostic and therapeutic difficulties. The aim of this review is to be an update of the specialized bibliography. Erythromelalgia is an infrequent episodic acrosyndrome affecting mainly both lower limbs symmetrically with the classic triad of erythema, warmth and burning pain. Primary erythromelalgia is an autosomal dominant inherited disorder, while secondary is associated with myeloproliferative diseases, among others. In its etiopathogenesis, there are neural and vascular abnormalities that can be combined. The diagnosis is based on exhaustive clinical history and physical examination. Complications are due to changes in the skin barrier function, ischemia and compromise of cutaneous nerves. Because of the complexity of its pathogenesis, erythromelalgia should always be included in the differential diagnosis of conditions that cause chronic pain and/or peripheral edema. The prevention of crisis is based on a strict control of triggers and promotion of preventive measures. Since there is no specific and effective treatment, control should focus on the underlying disease. However, there are numerous topical and systemic therapies that patients can benefit from.
The skin is an organ of singular structure with several complementary functions. As a sensory organ, it has a refined sensitivity to detect a great variety of stimuli of variable intensity. It is in charge of peripheral thermoregulation to avoid heat dispersion. All functions of the skin are performed through a close interrelation among its structures: epidermis, dermis and an abundant network of blood vessels innervated by sympathetic and sensory endings. The complexity of the skin makes it susceptible to sensitive neuropathies and sweat alterations associated with chronic intractable pain.1
Erythromelalgia (EM) is an episodic acrosyndrome that generally affects lower limbs bilaterally and symmetrically. The etymology of its name comes from the Greek: erythros “red”, melos “limb” and algos “pain”. During the crisis the skin becomes erythematous and warm, referred by the patient as a burning pain.2,3
The somatosensory system allows the perception of tactile, pressure, pain, and temperature stimuli, body position, movement and vibration. The somatic and sensory nerves are displayed in the skin, muscles, joints and fascia including different receptors such as nociceptor permitting the distinction between innocuous or potential damaging stimuli, sending signals to the spinal cord and brain for processing. The neuropathic pain is produced by alterations in the somatic sensorial system. EM is classified along with the chronic painful syndromes. The chronic pain is associated with multiple medical consultations and high prescription of drugs (Chart 1).3
The low prevalence of EM and it being an orphan disease pose diagnostic and therapeutic challenges. Mild or intermittent episodes could go unnoticed while the severe ones are difficult to control.
History and ClassificationEM was described in 1872 by Silas Weir Mitchell, and was known as Mitchell’s disease for a long time. A pioneer of pediatrics, Carl Jakob Gerhardt, published a paper in German in 1892, and the disorder was consequently named Gerhardt’s disease. In 1964, Babb described 51 patients and observed an increased incidence of the myeloproliferative disease associated with secondary EM. In 1990, Drenth and Michiels, from the Netherlands, proposed to name erythromelalgia whenever it is secondary to other disorders or even to drugs, while the idiopathic form should be named as erythermalgia. Recently, different groups of researchers described vascular and neural dysfunction in both primary and secondary forms. In 2004 EM became the first human disease associated with a sodium (Na +) channel mutation and neuropathic chronic pain.2,4-13
Clinical formsPrimary EM causes recurrent episodes characterized by painful bilateral neuropathy affecting limbs without other organic compromise, starting with intense pain and arterial hypertension. Feet soles and hand palms could be affected primarily. Its progression could attain a greater compromise of both upper and lower limbs. The frequency of sporadic episodes could become daily or even constant leading to incapacity.8,9
Primary EM is an autosomal dominant inherited disorder encoded by OMIN (Online Mendelian Inheritance in Man) as #133020. It is associated with an alteration on the α subunit protein of the sodium channel type 9 (SCN9A), affecting the Nav1.7 channel that is expressed mainly in dorsal root ganglia and the sympathetic ganglia neurons. The SCN9A gene mutation in the chromosome 2 (2q31-32) could be sporadic or familial. Another inherited form, not related to SCN9A, has been recently reported. Both must be differentiated from secondary EM, which is associated with systemic diseases, medications or toxins. The disease in children under 10 years old is mainly primary, sporadic and more rarely familial. In adulthood, it could be either primary or secondary.8-11 A child with a parent carrying the SCN9A mutation has a 50% probability to inherit the disorder. On the other hand, it has been seen that 7% of patients have a first-degree family member affected. The severity of the disorder is variable within the family members of the affected pedigree. This behavior cannot be explained up to now.5,12-15
The primary form could be classified according the age of onset as “early” if it appears in the first two decades of life or “late“. The age of onset of the symptoms has been correlated with Na+ channel voltage activation produced by pathogenic SCN9A variants. The late onset of symptoms could be associated with mutations producing less Na+ channel activation and lower neuron excitability.16-18
Symptoms
• Punctures, tingling
• Punctures and needles sensation
• Electric shock
• Warmth and burning sensation
Skin signs
• Erythematous or mottled skin
Clinical examination
• Allodynia produced by slight and innocuous contact
• Altered puncture sensation
Painful conditions of genetic origin include EM, paroxysmal extreme pain disorder, small fiber neuropathy and disautonomy, all related to SCN9A.16
Secondary EM could precede the underlying disease in at least two years and could be present in children as well as in adults. Myeloproliferative diseases, paraneoplasias, autoimmune diseases, toxins and infections are among the associated disorders. Myeloproliferative neoplasias, essential thrombocytopenia and polycythemia vera are the most frequent. The risk of secondary EM associated with myeloproliferative disease is 3% to 65%. Some authors consider myeloproliferative disorders and essential thrombocytopenia to be responsible for 20% of the cases in secondary EM. Previous studies propose that occlusion of the arterial microcirculation could be triggered by platelet activation and aggregation in these patients. High doses of aspirin are beneficial in these cases. EM can precede or be present at the same time as the underlying disease (Chart 2).2, 18-34
EpidemiologyThe prevalence of either primary or secondary EM is not well known, since population studies make no difference between them. A Norwegian report states a yearly incidence of 2/100,000, where primary EM accounts for two thirds of cases. In Sweden, the incidence was 0.36/100,000, while in the USA it was 1.3/100,000, with no difference between primary or secondary EM.19
Regarding sex, a 2-3:1 female/male ratio was observed. Davis et al.9 found 72.6% of females vs. 27.4% males in a Mayo Clinic sample of 169 patients, while Parker et al.18 found 41 females in a sample of 46 patients. No reasons were given to explain sex difference.1,35,36
The mean age of onset, regardless of the clinical form, is about 50 to 60 years of age.35,37 An epidemic presentation due to high weather temperature has been reported.38,39
PathophysiologyFirst of all, we must take into account some concepts about the physiology of pain. The pathologic mechanisms are complex; neural and vascular dysfunction might be present in primary and secondary EM.
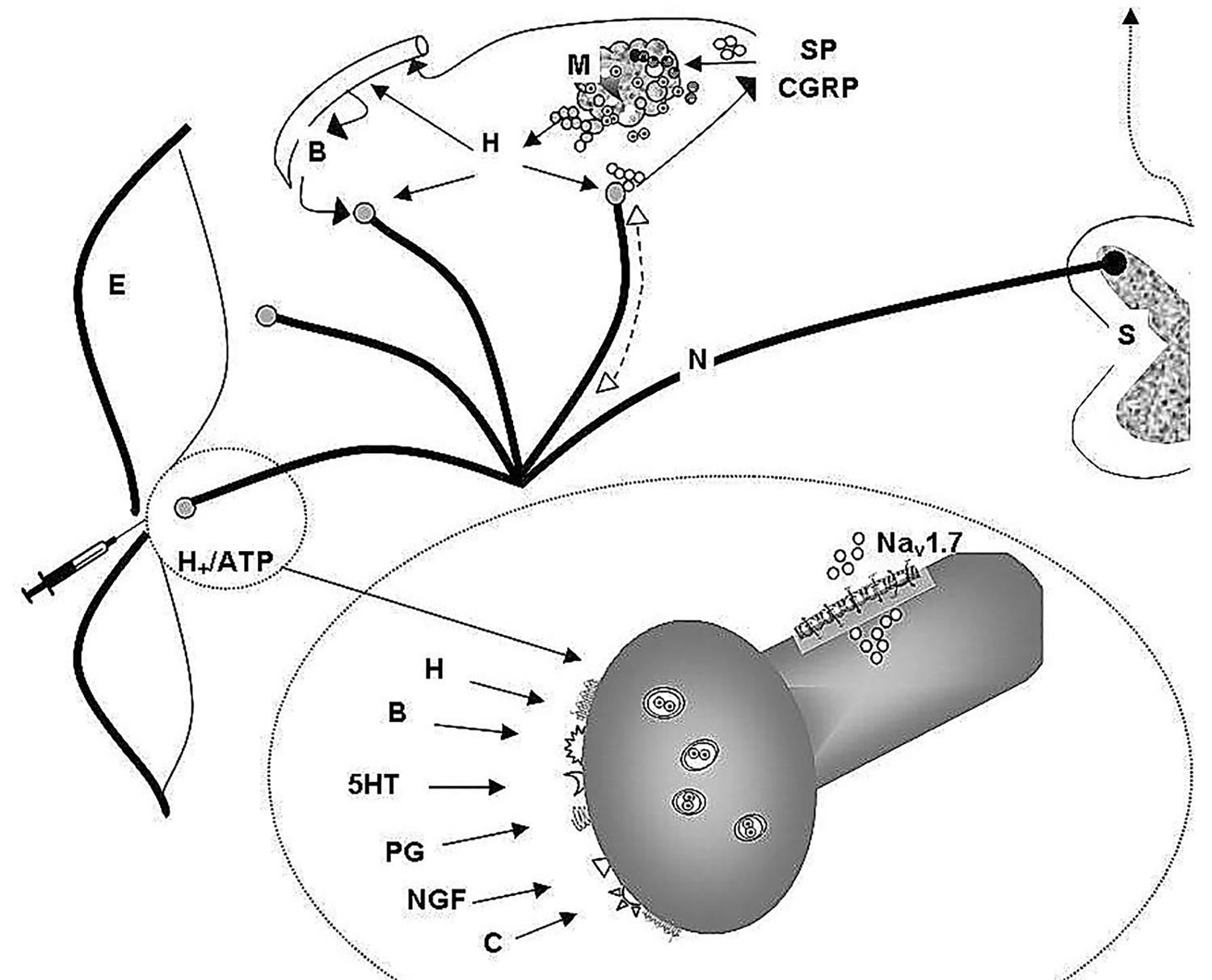
Free nerve endings of the skin are peripheral arborizations of a bipolar neuron whose neural body is located in the spinal node of the dorsal root of the spinal cord, where the first synapse occurs and the collected information is sent to the brain. These specialized cell structures act as nociceptors displaying also numerous biochemical receptors. The peptidergic nociceptor, besides transmitting afferences to the dorsal horn, have an efferent function, releasing substance P and a calcitonin gene-related peptide (CGRP) antidromically. Substance P and CGRP released at tissue injuries induce vasodilation, increase vascular permeability, releasing histamine and other local mediators (autacoids) from mast cells and other cells. Moreover, the peptidergic nociceptor has the tyrosine kinase A receptor for nerve growth factor and the transitory potential receptor of the channel type vanilloid 1 and ankirin 1.
The activation and modification of the receptor sensitivity are produced by changes on neural membrane of the sodium, potassium and calcium conductance. These changes can be produced acting directly on ionic channels or through opening of ionic channels associated with membrane receptors. The sodium channels controlled by voltage Nav are key determinants of the nociceptor excitability and play a fundamental role in pain transmission. The Nav1.7 it is expressed in the neurons of the dorsal cord ganglion including both the neuron peripheral endings (free neuron endings) and the central endings in the dorsal horn. The activation of the ionic channels produces the activation of the action potential and also the release of inflammation mediators.
In the inflammatory process, groups of active factors act, as follows:
- Hydrogen ions and adenosine triphosphate that exhibit a direct relation with the tissue lesion and activate the already excited nociceptor by the causal stimulus.
- Bradykinin, histamine, serotonin, prostaglandins, leukotriene, proinflammatory cytokines, nerve growth factor, all associated with the inflammatory process and sensitizing the nociceptor in order to respond to physical stimuli and to other active substances.
-Substance P, neurokinin A, CGRP released by the nociceptor.
The amplification of the message is assured not only by the release in the inflammatory injury but also through supplementary recruitment of the adjacent activated or sensitized fibers, mainly through the axonal reflex, e.g. the so called neurogenic inflammation. Consequently, the primary afferents contribute to this multifaceted inflammatory process, releasing neuropeptides that take part in a sort of “oil spot nociceptor sensitization.” (Figure 1).2,40-44
• Colagenopathies: Lupus erythematosus, Rheumatoid arthritis, Mixed connective tissue diseases,SjÖgren syndrome,Leukocytoclastic vasculitis
• Hematological Diseases: Polycythemia vera, Idiopathic thrombocytopenia, Leukemia, Spherocytic and pernicious anemia, Cryoglobulinemia, Systemic mastocytosis
• Drug Reactions: Iodinated contrasts agents, Calcium channel blockers (Nicardipine, Nifedipine, Felodipine) Cyclosporine, Rosuvastatine, Bromocriptine, topical Isopropanolol, Pergolide, Norephedrine
• Vascular diseases: Venous insufficiency, Thromboembolism,Arterial hypertension
• Neuropathies: Reflex sympathetic dystrophy, Small fiber neuropathies of several etiologies, Neurofibromatosis, Multiple sclerosis, Riley-Day syndrome
• Physical diseases: Trauma, Burn
• Intoxications: Mushrooms, Metals (plumb, mercury, arsenic)
• Neoplasias: Paraneoplastic syndrome, Colon and breast cancer, Malignant thymoma, Astrocytoma, Subcutaneous panniculitis-like T-cell lymphoma
• Miscellanea: Influenza and hepatitis vaccine, Amaurosis, Lichen sclerosus, Genetic diseases
Physiology of skin pain.
Cutaneous nociceptors play a fundamental role in the reception and transmission of painful stimulus. They are stimulated by different factors and in turn involved in neurogenic inflammation.
Notes: (S) dorsal root ganglia; (N) nociceptors; (SP) substance P; (CGRP) Calcitonin gene- related peptide; (H) histamine; (M) mast cells; (NGF) receptor for nerve growth factor; (H+/ ATP) Hydrogen ions and adenosine triphosphate; (B) bradykinin; (H) histamine; (5-HT) serotonin; (PG) prostaglandins; (C) proinflammatory cytokines; (E) epidermis; (Nav) voltage-gated sodium channel
Sensorial peripheral alterations, adrenergic dysfunction and distal neuropathy of small fibers might be present in EM. The damage to the nociceptor could produce chronic pain leading to phantom pain if the damaged nociceptor starts randomly triggering other nerves.45-48
In EM A-Delta afferent and C efferent sympathetic fibers could be damaged. Nevertheless, the nerves with greater caliber could be intact and consequently conduction velocity is normal. The small fibers are part of the autonomous neural system controlling sweat, heart rate and blood pressure. Small fiber neuropathy involves preferentially the axon terminals of long neural nerves affecting frequently the feet.49,50
The altered electric excitability of neurons can increase pain. In primary familiar and sporadic EM cases there is an alteration of the alpha subunit of the Nav1.7 sodium channel due genetic mutation. This mutation produces hyperexcitability of the sensorial peripheral and sympathetic neurons, decreasing the threshold and thus increasing the discharge frequency of the Nav 1.7 mutant of neural ganglions of dorsal roots. In these patients, C fibers have low conduction velocity, spontaneous activity and sensitization. The threshold activation of C fibers could occur at relatively low temperatures (32-36°).
Besides, C fiber activation produces vasodilation through the axon. The pain due to small fibers and C-nociceptor produces typical symptoms such as aching feet, burning pain and puncture or a cutting knife sensation. Tests of sweating, blood pressure and heart rate control in EM patients have revealed abnormalities in more than 80% of these patients, reflecting dysfunction of small nerve fibers. These patients also have low pain threshold suggesting this type of neuropathy. The cooling of limbs reduces the nociceptor threshold thus improving symptoms.9,42,45,46,51-53
Vascular disordersAround 80% of peripheral circulation flow through direct arteriovenous anastomoses, and 20% through capillaries. In EM there is a dysfunction of the microcirculation that causes the symptoms. Physical activity and high temperature increase the blood flow. The increased perfusion of the affected limb is paradoxically associated with tissue ischemia due to an abnormal distribution of the microvascular flow through arteriovenous pre-capillary shunts. The opening of arteriovenous anastomoses with the consequent flow increase leads to a decrease of the capillary circulation, diminishing the oxygenated blood and producing hypoxia and tissue pain.49,54-57
These patients have a low basal skin perfusion in comparison with normal controls. The acral skin shows less vascular constriction in response to the reflex stimulus of the adrenergic vasoconstrictor system. The temperature increase produces a greater decrease of flow due to an impairment of the vasodilatation mechanism mediated by the sensory nerves, reduced vascular density and the presence of arterial venous shunts or all these alterations combined.58
Interaction of both mechanismsThermoregulatory impairment of sweat glands in these patients is influenced by neural and vascular mechanisms. The diagnosis of small fibers neuropathy is observed in most of these patients. Whenever the nutritive perfusion is affected the skin thermoregulation is also altered, thus 88% of EM patients present some type of sweat impairment. The most frequent is anhidrosis or hypohidrosis of the distal type, followed in frequency by the global type; this covers 80% of the body surface. The most affected EM zones are generally anhidrotic.1,49,53-56
Moreover, since both neural and vascular dysfunctions are proximal and interconnected, they could interact. There are authors that propose two theories: A- The primary dysfunction of the small fibers could enhance an altered distribution of the microcirculation and hypoxia. B- The impaired vascular distribution leading to hypoxia could trigger the neuropathy.52,57,58
Other authors point out to both types of dysfunctions since they are exposed to the same pathological mechanism. As above mentioned, the altered activity of the sodium channel Nav1.7 is present in both sympathetic and sensory nerves. It has been recently demonstrated that sodium channels are present also in skin myocytes and vascular endothelium of arterioles and arterialvenous shunts. Other authors consider that both dysfunctions are exposed to the same pathological alterations. Since the altered activity of Nav1.7 sodium channels is present in both sympathetic and sensory nerves, and it is known that in the innervations of these vessels both nerves converge, these complex interrelations may contribute to produce skin erythema and pain in EM.1,9,58
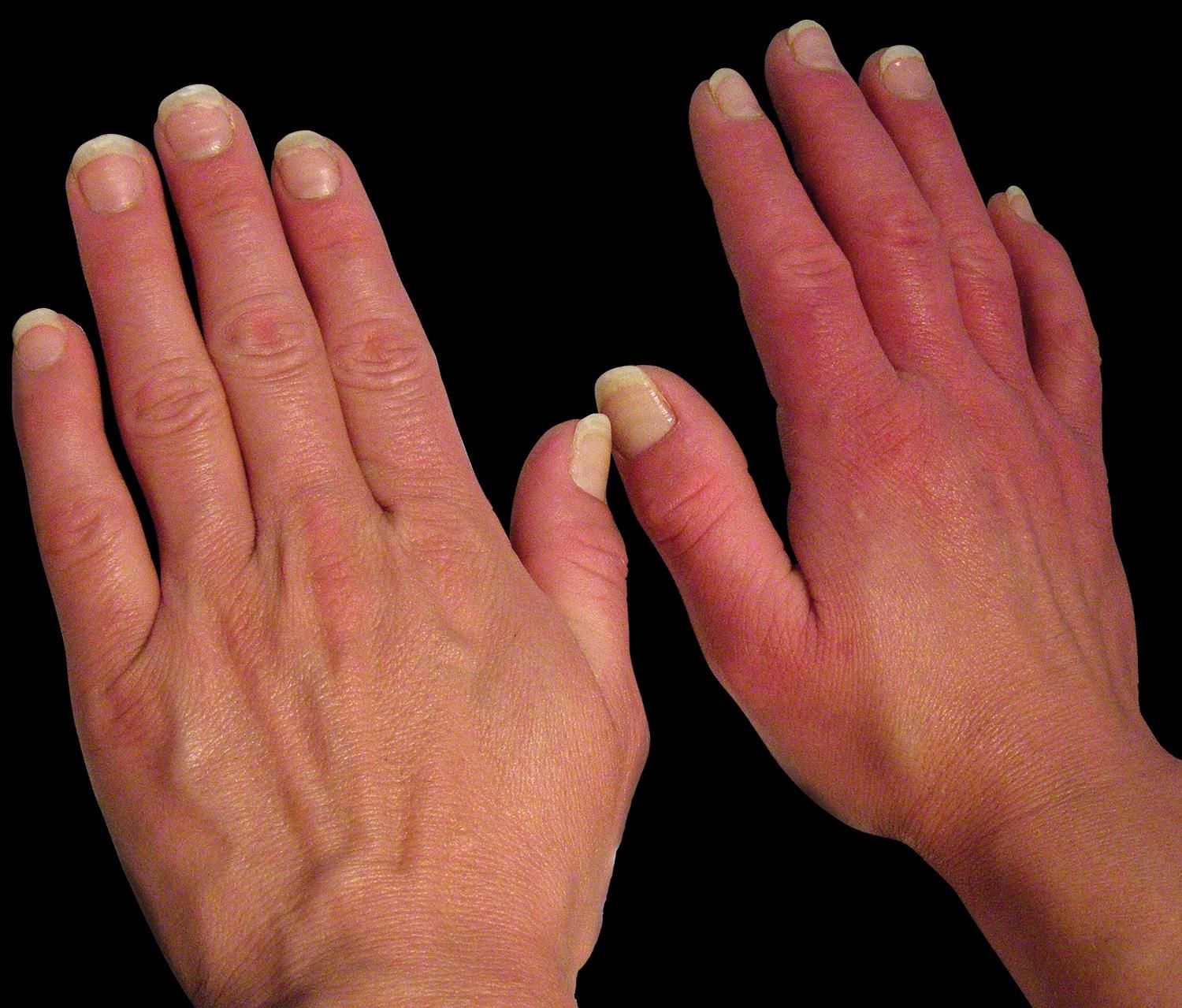
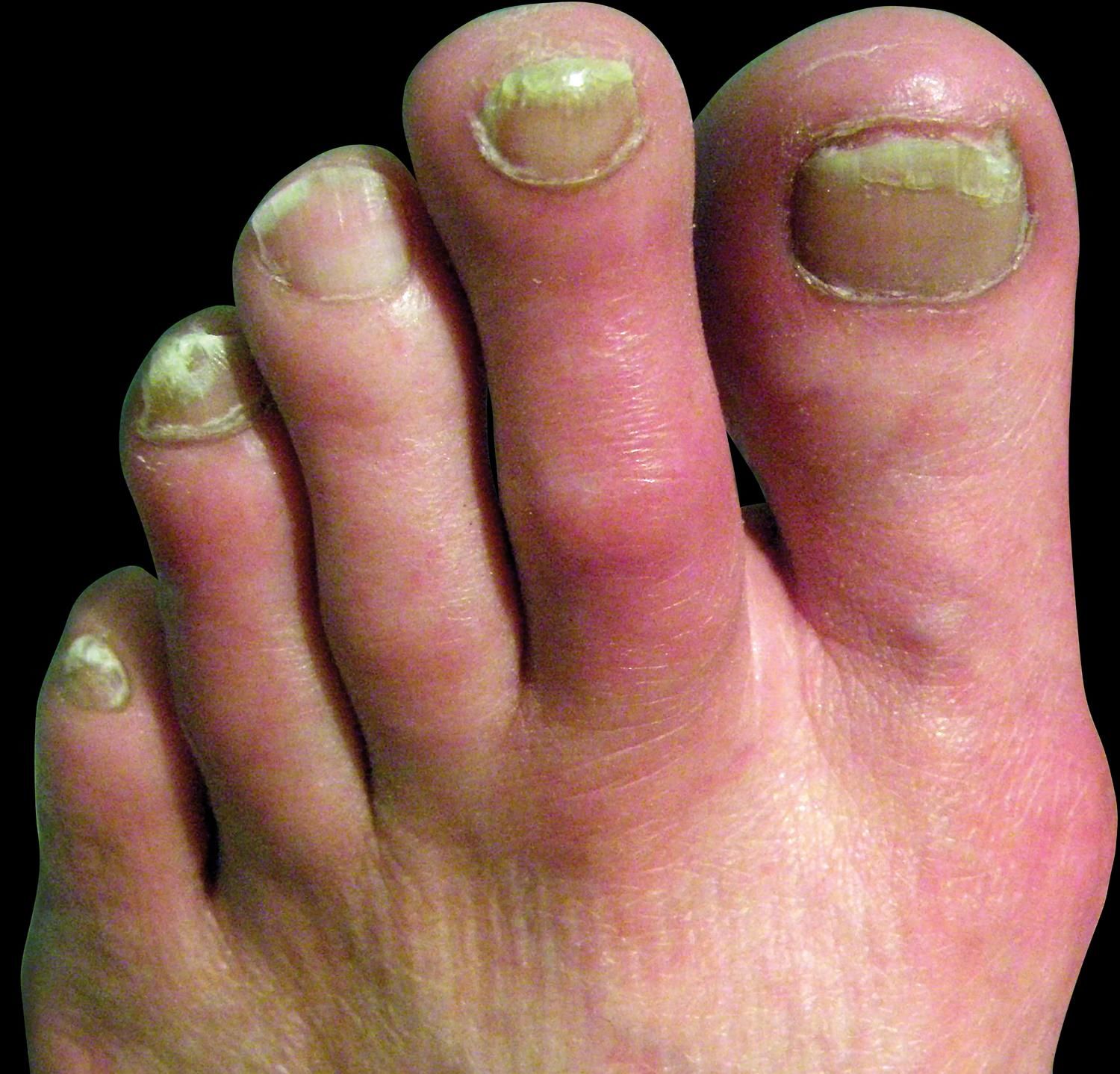
Clinical FeaturesPrimary and secondary EM are characterized by episodes of erythema, warmth and burning sensation of the limbs. The erythema could be mild to moderate with clear or ill-defined limits (Figures 2-3). During the episode, the warmth sensation is perceived by the patient and confirmed by the clinician. Pain is described by the patient as a burning sensation or as an electric shock or a throb. Some patients refer allodynia and/or hyperalgesia while others complain from itch. Edema and numbness in the limbs are observed in a low frequency. Parker et al. described the more frequent symptoms as follows: burning (96%), warmth (93%), pain (87%), redness (83%), inflammation (65%) and numbness (54%). The compromise could be bilateral and symmetric, but it could also be unilateral. The extension is variable from the terminal phalanx of the toes to the whole limb. The skin frequently presents distal anhidrosis or hypohidrosis.2,18,52,58-62
All the circulatory regions of the extremities could be affected, either single or multiple. In a great deal of reports, more than 80% of the lower limbs are affected, in contrast with 25% of upper limbs. They were also described in the face, ears and tongue. The episodes are intermittent or rarely continuous, lasting 2-3 hours. In the interval between episodes, the limb could be normal or cyanotic, with cold skin and numbness. Sometimes livedo reticularis could be present (Figure 4).2,18,63-66
The prevention is based on a strict control of triggering events, i.e. heat exposure or pressure, standing position for a long time, physical activity, emotions, and/or stress, alcohol ingestion, spicy food, vasodilator drugs, night time, exposure to chronic vibration or even light skin contact in severe cases.8,9
Symptoms could be improved with cold water or ice contact and raising the limb, nevertheless there is no improvement with analgesics.2,18,62,66
Regarding evolution, Davis et al. reported that after an eight-year follow up, 31% of patients exhibit deterioration, 25% had no change, 30% improved and 10% had a complete remission. There is a tendency for chronicity, with sleep and appetite disturbances, as well as anxiety and depression. The patients use open shoes and sleep with uncovered feet even in cold weather. They cannot walk long distances or be in a standing position for long time, nor practice any sport or dance. Due to these symptoms, both children and adults loose days of study, sport or work with resentment of their ludic and social activity. Due to their condition, they are prone to accidents at work and at home. If the episodes continue for a prolonged time, bed rest is recommended, otherwise a wheel chair must be considered. The affected quality of life could become a disability. Morbidity and mortality rates are higher in these patients in comparison with individuals matched by sex and age in the general population.2,4,5,18
ComplicationsComplications are related to the impairment of the barrier function, ischemia and neuropathy. The most frequent is secondary infection followed by fissures, blisters, painful ulcers, cyanosis, gangrene or necrosis. Many patients know that burning pain is relieved with cold water or ice, an electric fan or staying in places with low temperature. Some of these methods lead to skin maceration followed by epidermis slough thus increasing the damage to the skin barrier. Cold could trigger long lasting vasoconstriction provoking ischemia and tissue necrosis, what could be interpreted by patients as aggravation of the disorder. Cutaneous dystrophies could increase pain and at the same time slow skin repair, deteriorating their quality of life and leading to social isolation, and in case of hypothermia adding a life-threatening risk. The patient must be advised to discontinue these practices. Some patients could develop skin automutilation behavior; although its mechanism is unknown, the poor quality of life could be related to this condition.2,18,32,34,36,62,66
Frequent comorbiditiesEven having already discussed the etiology of secondary EM, certain frequent comorbidities deserve a comment. Comorbidities must be taken into account since they increase hospitalization, increase medication taking, and mortality rate, thus worsening the perception of quality of life of these patients. Davis et al. report the following comorbidity percentages as follows: smoking (50%) hypertension (13.7%), hyperlipidemia (11.3%) and diabetes mellitus (2.4%).2,26,27,29,35-37,65,67
The functional acrosyndrome is closely related. The presence of erythema pernio and in second place, livedo reticularis, is frequent in patients with acrocyanosis, erythromelalgia and less in cases of Raynaud`s disease. Both Raynaud and EM are microvascular disorders with local alterations or impaired thermoregulation that could be due to interrelated underlying mechanisms. Heidrich confirmed the coexistence of EM, acrocyanosis and Raynaud.4,37,68
Small fiber neuropathy is considered as an early disorder in painful diabetic neuropathy that contributes to the presence of distal ulcers. Moreover, it might be an interaction between the latter neuropathy and the microcirculation in these patients. The diabetic microangiopathy and neuropathy could add up to the EM with an overlapping of signs and symptoms generating difficulties in the diagnosis and also complicating the disorder.42,46,47,69-71
Some authors report the presence of headaches in these patients. This symptom is common in patients with polycythemia vera associated with erythromelalgia as the principal disorder. Nevertheless, no relationship between the etiopathogenesis of EM and headache have been described up to now in the literature.72,73
Diagnosis and Differential DiagnosisThere are controversies regarding the accepted diagnostic criteria since not all cases are included. While pending new diagnostic criteria, we recommend that accurate and detailed questioning be performed (Chart 3). The physical examination of the skin, can present acrocyanosis, telangiectasias, vascular dilations and red nails (Figure 5).
There are presently non-complementary medical studies to attain a precise diagnosis. A complete blood cell count with normal platelet count rules out a probable associated myeloproliferative disease. Skin biopsy is not specific for EM, even if there could be histological evidence of decreased small fibers density there is not a clearly established relationship with neuropathy.3,74-77
The positive test using warmth exposure is frankly positive when the patient describes the symptoms at 32°-36°C range. Thermography usually shows an increase in temperature in the affected areas during episodes. This test could be useful regarding the differential diagnosis with the complex regional pain syndrome. The neurological study and the electromyography are indispensable to rule out peripheral neuropathies of other etiologies.20,29,66
A complete clinical study of the patient would make possible to discriminate between a primary or secondary disorder rendering easier the differential diagnosis. Due to the complex pathogenesis of the diseases with chronic pain and/or peripheral edema the differential diagnosis is important (Chart 4).29,72,77
TreatmentPreventive measures should be taken into account. Peripheral vasodilator drugs are contraindicated as well as exposure to excessive warmth, intensive physical exercise, smoking, and physical or psychological stress. Comfortable shoes and appropriate to weather or temperature are indicated, as well as physical rehabilitation and psychic support. Physical exercise without impact 2 or 3 times weekly should be recommended and also yoga and swimming.77,78
• Family history
• Frequency of occurrence
• Relation with triggers: cold, warmth. emotions, physical activity, humidity
• Duration and characteristics of the episodes
• Approximate triggering temperature
• Symptoms and signs diminish with cold or warmth?
• Color change of limbs depending on their position
• Current medication indicated by a professional or not
• Unhealthy habits: smoking, alcohol, social drugs
• Work and sport activity
Since there are no specific and effective treatments, the main primary disorder treatment that ameliorates the symptoms should be proposed. Acetylsalicylic acid is useful in EM secondary to polycythemia vera or others hematological entities. Aspirin prevents platelet aggregation through irreversible inhibition of cyclooxygenase in doses of 325 to 650mg/daily. A 500mg dose could be administered during 3 days. This treatment is also pathognomonic since it could be used as a diagnostic test in EM associated with proliferative syndromes. The maintenance dose prescribed is 75 mg/daily. Nevertheless, not all patients respond to aspirin treatment. Thus, EM secondary to polycythemia vera can respond to aspirin associated with hydroxyurea while in other patients, alpha-2b interferon and epidural analgesia are necessary.20,28,29,79,80
It has been recently described a subgroup of EM patients responding to systemic corticoids. Thus, it is postulated that in EM cases associated with infections, surgery, trauma or subacute diseases, high or very high systemic corticosteroid doses could be effective. A high dose is considered 40 mg of prednisone or higher, or its equivalent, administered daily during 5 or more days. The medication must be given early, before the installation of irreversible damage of the nociceptor. The time of treatment is dependent of the symptoms evolution.81
There are alternative therapies either topical applications or systemic ones (Chart 5).43,81-95
Topical applications are currently in development considering the interrelations between non-neural cells in the skin and free nerve endings. Some authors argue that with topical therapy the magnitude of pain relief in the patient cannot be predicted, nevertheless is a more secure option and free of serious side effects. Others propose to combine the analgesic topical preparations associated with anti-inflammatory drugs. Regarding useful components, lipophilic and hydrophilic ones are recommended. It is also proposed the use of liposome as drug delivery. The most used are lidocaine and capsaicin. Other options are analgesics such as ketamine, amitriptyline, and phenytoin as cream, gels, or ointments. Phenytoin cream acts on the voltage regulated sodium channel. There is a good response with a topical preparation containing 0.2% midodrine, which is an alpha agonist. The role of autacoids, such as palmitoylethanolamide as restorer of the neuroimmunologic homeostasis is under evaluation43,82-84,96
Moreover, topical lidocaine inhibits the voltage regulated neuronal sodium channel, thus stabilizing the neuron membrane potential of the hyperexcited peripheral nerve fibers. This mechanism diminishes both allodynia and hyperalgesia. Its presentation as patch is disadvantageous when applied to feet.82-83
• Limbs with severe pain due to trauma or infection
• Recovery phase of freezing
• Recovery phase of erythema pernio
• Paroxysmal painful disorder
• Antiphospholipid antibody syndrome
• Thrombophlebitis obliterans
• Peripheral neuropathy
• Drugs edema
• Peripheral vascular disorders
• Hand-Shoulder syndrome
• Lipodermatosclerosis
• Multiple sclerosis
• Plantar fasciitis
• Reflex sympathetic dystrophy syndrome
• Redness phase of Raynaud’s phenomenon
• Acute gout
• Bacterial cellulitis
• Vasculitis
• Arteriosclerosis obliterans
• Diabetic neuropathy
• Edema due to macro and/or microcirculation impairment
• Acrocyanosis
• Metatarsalgia
• Fabry disease
• Paraneoplastic syndrome
• Restless legs syndrome
Different concentrations of topical capsaicin are useful in the peripheral neuropathic pain since it is an agonist of the vanilloid receptor. The first application triggers a sensitivity that decreases progressively to attain a persistent desensitization. Capsaicin depletes and prevents the accumulation of substance P in peripheral sensory neurons.85,86
Systemic regulation of sodium channels could be obtained through several pharmacological agentes, i.e,. anesthetics, antiarrhythmic drugs and anticonvulsants, among others. Lately, it has been reported that EM patients carrying the S241T mutation on the Nav1.7 sodium channel could benefit with oral carbamazepine that acts on cerebral pain regulation.94
Among efficient therapies on microcirculation, peripheral vasodilators are restricted. Buflomedil is indicated during long periods of cyanosis and coolness, since it opens the pre-capillaries sphincters restoring the microcirculation and through their hemorheological effect increases the erythrocyte membrane elasticity. These actions improve the blood flow, increasing tissue oxygenation turning it into a valid therapeutic option in EM. The addition of two or more therapeutic agents in lower dose could enhance their action and at the same time reduce side effects.58,77,90
TOPICAL ADMINISTRATION
• Lidocaine patch 5%: Diurnal application (12h). No nocturnal application
• Gel combined amitriptyline 1% associated with ketamine 0.5% : Application 4-5 times daily
• Capsaicin 8%: Unique application patch. Probable effect up to 12 weeks
SYSTEMIC ADMINISTRATION
• Corticosteroids: High or very high doses are recommended for at least 3 months. 40mg/d or higher
• Oxcarbazepine: Adults starting dose: 300mg/d and increasing to 900mg/d or higher, control evolution. Children starting dose: 10mg/kg per body weight, daily with gradual increase. Maintenance dose around 30mg/kg / body weight.
• Amitriptyline: 10mg/d; increasing 10mg/week. Dose range 75–150mg/d
• Gabapentin: Optimal dose: 900 to 1800mg daily, administered in 3 doses. Starting with 300mg on day 1, increasing 300mg daily to attain optimal dose: 2400mg- 3600mg /d. Well tolerated.
• Pregabalin: Starting dose 75 mg 1 or 2 doses/d, increasing until 150mg each 12h. It could be increase to attain 600 mg/d
• Flecainide: Starting dose 100mg each 12h
• Mexiletine (Na+ channel blocker): 100mg each 8h; afterward 200mg each 8h. Range dose: 600-1200mg/d
• Buflomedil: Adults: oral dose 300-600mg/d
• Magnesium: Magnesium citrate 528mg BID
Cyproheptadine and antihistamines such as pizotifen and paroxetine have also been evaluated. Magnesium in high doses decreases pain.18,32,95
Ziconotide has been used for the amelioration of chronic pain, although its mechanism in human beings it is not known. In animals, it blocks the type N calcium channels in the primary nociceptive nerves in the spinal cord. The main disadvantage is the intrathecal administration. The selective sodium channel blocking drugs are proven in clinical trials. The results obtained might be satisfactory due to their molecular effect. The BIIB074, a sodium channel blocker, is in Phase 1 trial in patients with trigeminal neuralgia with promising results in EM patients. In refractory cases prostacyclin infusion and sympathetic blocking of the affected zone has been reported.97-101
Final CommentsThe EM is a painful chronic skin syndrome posing multiple challenges related to its diagnosis, physiopathology and treatment. Dermatology is enriched through the molecular research of the neurobiology of chronic pain.
Financial support: None.
Conflict of interest: None.