Polypoid melanoma is a variant of nodular melanoma, whose poor prognosis depends on its thickness and the presence of ulceration at the time of diagnosis. The authors report two cases of polypoid melanoma, presenting as broad, cauliflower-like, polypoid masses. Dermoscopy was characterized by a multicolored pattern, atypical polymorphic vessels, and the fiber sign. Clinical and dermoscopic features can help to diagnose polypoid melanoma and exclude other possible differential diagnoses. However, histology remains mandatory to confirm the diagnostic suspicion.
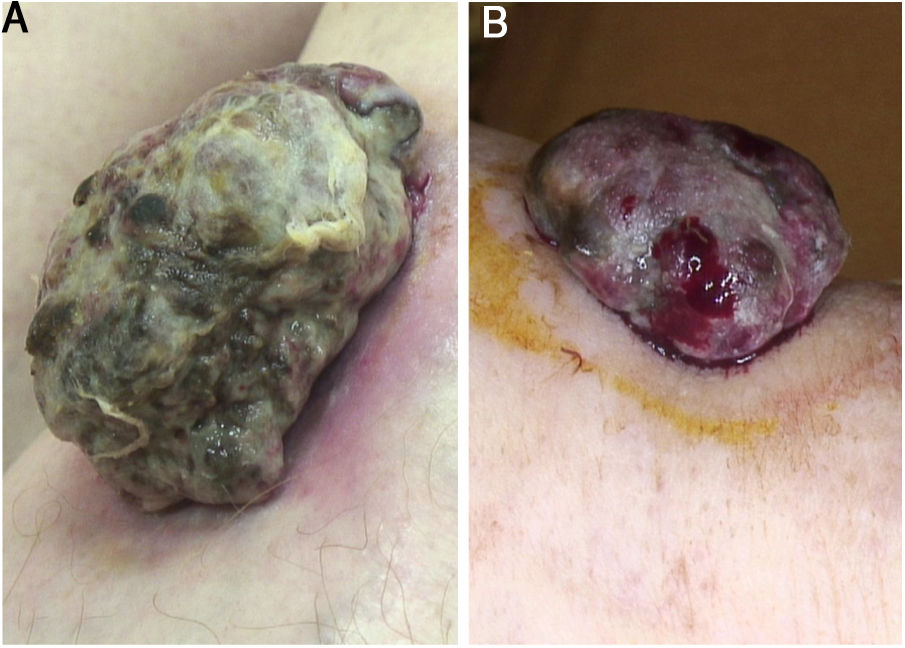
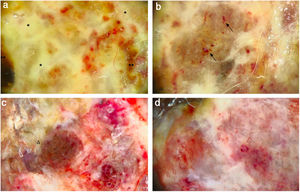
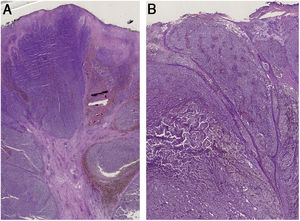
A 59-year-old woman was referred to this service for an exophytic mass located on her left leg, of six months duration. On clinical examination, the lesion was 1.8×4.5cm in its greatest dimensions, showed necrotic-hemorrhagic and fibrinous areas, and deformed the underlying erythematous skin (Fig. 1A). Dermoscopy revealed a multicolored pattern with a diffuse yellow background and irregularly distributed red, brown, and gray areas. Observation of atypical polymorphic vessels was impaired due to serohematic crusts and gauze filaments that were trapped in the irregular surface of the lesion (Fig. 2A and B). Histopathology showed an aggressive polypoid melanoma (PM) with a Breslow's depth of 12mm, ulceration, 3–6mitoses/mm2, lymphovascular involvement, fibroepithelial branches that subdivided the tumor into lobules, and pleomorphic vessels (Fig. 3A). Although nodal involvement was positive, no distant metastases were found. Anti-PD-1 therapy was initiated. During the first year of follow-up, there was no instrumental evidence of recurrence.
(A) Sessile nodular mass, 1.8×4.5cm on its greatest dimension, surrounded by an erythematous halo on the anterior area of the leg. Necrotic and fibrinous areas and gauze filaments incorporated within the tumor surface can be observed. (B) Broad-based sessile nodule (1.5×3.8cm on its greatest dimension) on the anterior area of the leg, deforming the underlying skin. Necrotic and fibrinous areas and bleeding are evident.
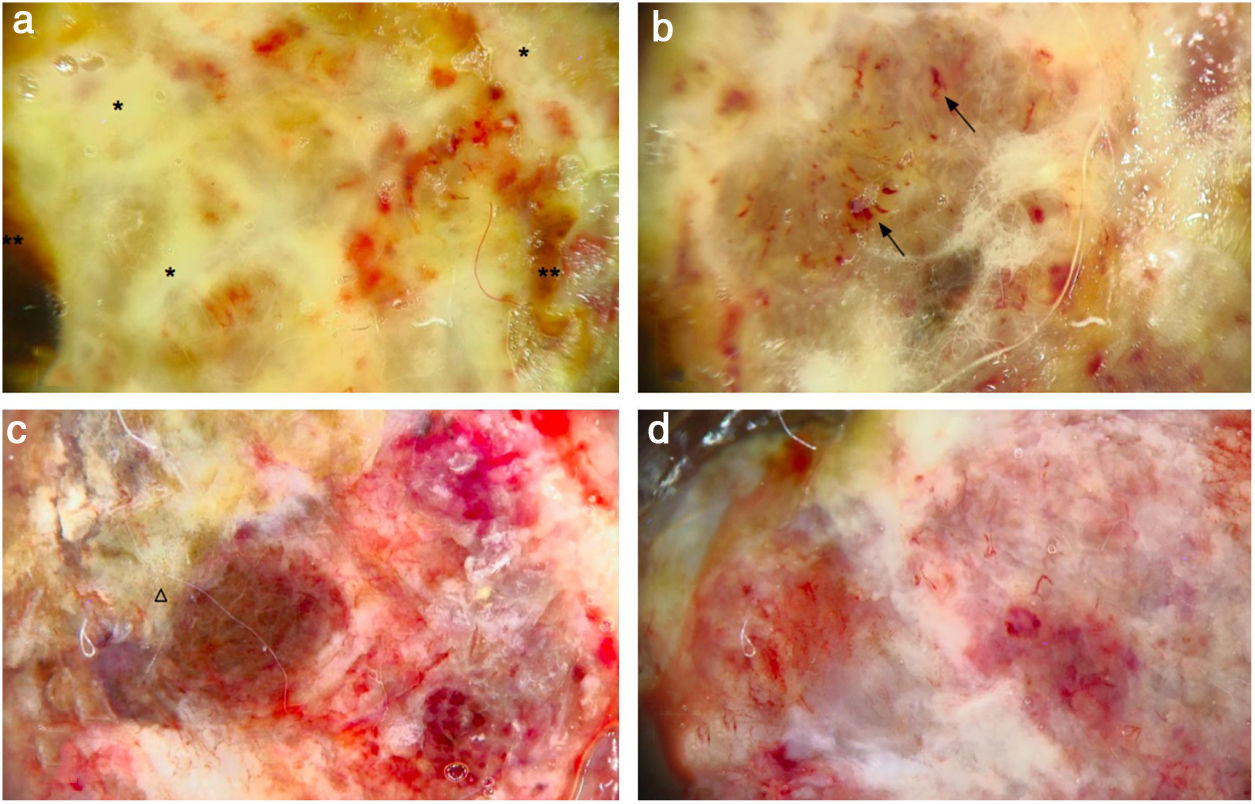
(A) Dermoscopy shows a multicolored pattern, with a diffuse yellow background (*) and some irregularly distributed red, brown, and gray areas (**). “Fiber sign”: observation of the atypical polymorphic vascular component is hindered by gauze filaments trapped into the irregular surface of the tumor. (B) Vessels appear atypical, polymorphic, and mainly dilated (arrows). (C) Dermoscopy reveals a multicolored pattern, with a diffuse red and white background and some yellow, brown, and gray areas irregularly spread on it (triangle). (D) Atypical vessels over a red and white background are clearly visible.
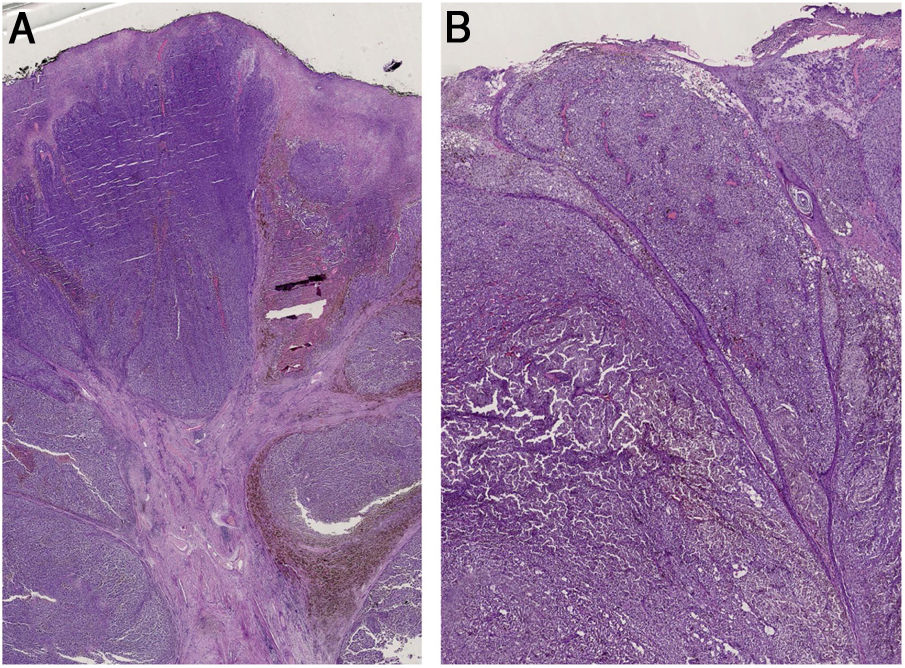
(A) Histopathology shows fibroepithelial branches subdividing the tumor into lobules. Pigment distribution is irregular, both in terms of quantity and depth. Pleomorphic vessels and ulceration are visible throughout the lesions. (B) Histological findings include ulceration, atypical polymorphic vessels with scattered depositions of pigment, and absence of rete ridges. Inflammatory infiltrate is scarce.
An 82-year-old Italian woman was referred to this service due to a six-month-history of a nodular mass on her left leg. On physical examination, the lesion was sessile, had broad dimensions (1.5×3.8cm), with an irregular crusty bleeding surface. The lesion deformed the underlying skin and was surrounded by an erythematous halo (Fig. 1B). Dermoscopy revealed polymorphic vessels and a multicolored pattern, with a diffuse red and white background and some irregularly spread yellow, brown, and gray areas (Fig. 2C and D). Histopathology showed a Breslow's depth of 10mm, ulceration, >11mitoses/mm2, lymphovascular involvement, atypical polymorphic vessels with scattered depositions of pigment, absence of rete ridges and scarce inflammatory infiltrate (Fig. 3B). The patient, who was staged as IIIC melanoma given the positive nodal involvement and negative distant metastases, was referred to another hospital, closer to her abode.
In both cases, molecular analysis showed negative BRAF mutation (pyrosequencing of exon 15) and positive NRAS mutation in exon 3 (pyrosequencing of exon 2 and 3).
DiscussionPM is characterized by an irregular surface and cauliflower-like profile.1–3 Although PM typically affects the mucosae, when involving the skin, it usually affects the back.2,3 Histologically, PM shows marked cytological atypia, nuclear pleomorphism, and a plentiful mitotic count.3,4 The initial radial-horizontal growth phase rapidly evolves into a nodular-vertical phase, with an important risk of vascular embolism.2,3 To date, there are few reports of the use of dermoscopy on PM. Hikawa et al. reported a case of PM overlapping a superficial spreading melanoma with an irregular multicomponent pattern, characterized by the presence of an atypical network, globules, and blotches.1 Cabrera et al. described a case of PM showing a blue-white veil in the exophytic part of the lesion and big blue-gray nests and whitish areas at the base of the stalk.5 Accordingly to Hikawa et al., a clear dermoscopic analysis of the tumor can be impeded by the presence of crusts and fibers of clothing, gauzes, or patient's own hair, the so-called “fiber sign”, that is an indirect dermoscopic clue of ulceration.6
To the best of the authors’ knowledge, this is the first report to find not only similar macroscopic clinical findings and the same unusual localization, but also similar dermoscopic features: in both cases, the tumor presented with an irregular crusted-fibrinous cauliflower-like surface and some bleeding areas, imprinting the underlying erythematous skin, as in the case reported by Pérez-Wilson et al. The most relevant dermoscopic finding of the present cases of PM were the atypical polymorphic vessels and the multicolored pattern: the yellow color was mainly related to the presence of fibrin; the brown, black, and red background were associated with the “true” surface of the tumor, whereas the whitish streaks were due to the fibrous components of the tumor.
Dermoscopy can be useful for the differential diagnosis of PM with other malignant entities, even if it may be challenging. Poorly differentiated squamous cell carcinoma shows a predominance of red color, erosion/ulceration, and residual white structures.7 Pigmented squamous cell carcinoma is characterized by homogeneous grey-blue pigmentation, ulceration, radial streaks, and globules.8 The main dermoscopic features of Merkel cell carcinoma include milky red areas along with polymorphous, shiny white areas within the body of the tumor, as well as linear, irregular, and arborizing vessels.9
Histopathology remains mandatory for a definitive diagnosis of PM. The lumpy cauliflower-like appearance, as well as the whitish streaks of the present two cases of PM were due to the presence of fibroepithelial branches that subdivided the tumors into lobules, whereas the multicolored pattern represented the dermoscopic counterpart of the irregular pigment distribution on histology.
Both cases showed NRAS mutation; this finding is in accordance with the literature, since NRAS mutations are reported to be associated with nodular melanoma and localization on the limbs.10
Financial supportNone declared.
Authors’ contributionsAmbra Di Altobrando: Approval of the final version of the manuscript; design and planning of the study; drafting and editing of the manuscript; collection, analysis, and interpretation of data; effective participation in research orientation; intellectual participation in the propaedeutic and/or therapeutic conduct of the studied cases; critical review of the literature.
Annalisa Patrizi: Approval of the final version of the manuscript; effective participation in research orientation.
Emi Dika: Critical review of the manuscript.
Francesco Savoia: Approval of the final version of the manuscript; design and planning of the study; drafting and editing of the manuscript; collection, analysis, and interpretation of data; effective participation in research orientation; intellectual participation in the propaedeutic and/or therapeutic conduct of the studied cases; critical review of the literature; critical review of the manuscript.
Conflicts of interestNone declared.
Special thanks to Dr. Cosimo Misciali and Dr. Carlotta Baraldi for the histologic images.
How to cite this article: Di Altobrando A, Patrizi A, Dika E, Savoia F. Cauliflower-like exophytic mass on the skin: polypoid melanoma. Clinical, dermoscopic, and histologic features. An Bras Dermatol. 2020. https://doi.org/10.1016/j.abd.2020.04.010
Study conducted at the University of Bologna, Bologna, Italy.