Dear Editor,
A 29-year-old female patient, received a dose of the yellow fever vaccine, developing headache, oral ulcers, and bilateral ocular erythema, edema and pruritus 4 days later. She sought medical attention and was instructed to use eye drops containing polymyxin B, neomycin and dexamethasone. The following day, she presented worsening of symptoms, with fever, decrease in general clinical condition, and brownish-erythematous macules on her hands. She was admitted to the Emergency Care Unit, remaining hospitalized for three days, when dipirone, diclofenac and systemic dexamethasone were prescribed.
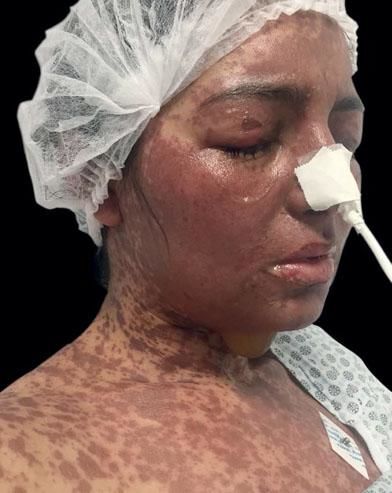
Due to the worsening of the clinical condition, the patient was transferred to our hospital on the eighth day after vaccination, with generalized confluent brownish-erythematous macules and plaques, blisters, and epidermal detachment on the back and face (Figure 1).
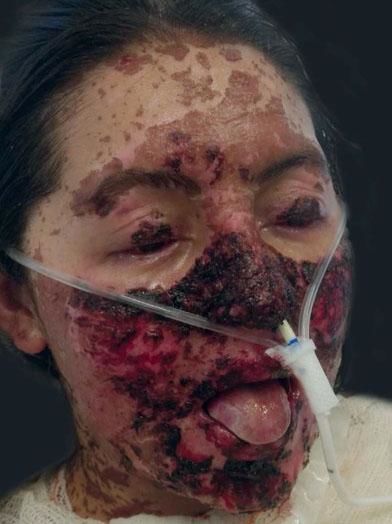
After being diagnosed with toxic epidermal necrolysis (TEN), she was promptly admitted to the Intensive Care Unit (ICU), and IV human immunoglobulin was introduced at a dose of 3g/kg (180g administered over 5 days), prednisone 20mg/day for 5 days, and acetaminophen 500mg if needed. She was maintained under contact isolation precaution, with nasogastric tube feeding, mucosal humidification, and occlusive bandages with Vaseline®. In the following days, she presented detachment of nail plates of her fingers and toes, and cutaneous necrosis on the face (Figure 2). She evolved with progressive improvement of clinical, ophthalmologic and dermatological conditions.
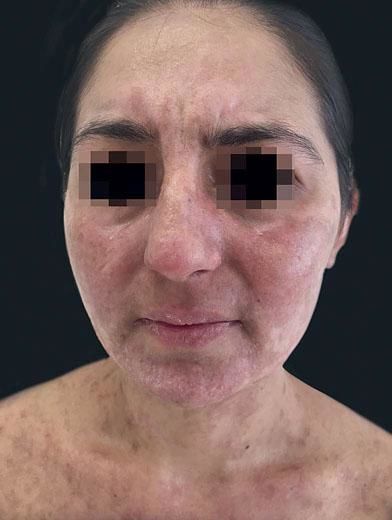
She was discharged after one month, with cutaneous reepithelialization, generalized residual hyperchromic macules (Figure 3) and granulation tissue in the nail folds. The patient persisted with decreased visual acuity, maintaining follow-up with ophthalmology.
Adverse drug reactions (ADR) are cutaneous manifestations that can occur after the use of any chemical by any route of administration. The best known are erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), considered by some authors as a spectrum of the same disease.1 ADR occur mostly after use of medications such as anticonvulsants, neuroleptics, antibiotics, antifungals, diuretics, non-hormonal anti-inflammatories, and analgesics. EM was reported after HPV and meningitis vaccines, and its onset after vaccination generates contraindication to new doses, due to the risk of SJS and TEN.2,3 Although rare, there are reports of TEN induced by vaccines such as triple viral, triple bacterial, influenza, polio, and BCG.4
TEN is characterized by the detachment of the epidermis (> 30% of the body surface) due to necrosis.4 The clinical manifestations occur 4 to 28 days after the introduction of the chemical substance. Prodromes may occur, such as fever, odynophagia, cough, ocular burning, and oral mucositis. The cutaneous lesions are erythematous macules with poorly defined contour and purpuric center, usually starting on the face and then spreading down the neck and torso to the arms, legs, and feet. TEN can affect the nails, causing onycholysis and subsequent shedding.1 The insult at the dermal–epidermal junction generates flaccid blisters that break easily, resulting in extensively denuded and friable dermis, with positive Nikolsky’s sign. Stomatitis, balanitis, colitis, conjunctivitis and blepharitis may occur, as well as involvement of the respiratory and gastrointestinal tracts.
Treatment should occur in an ICU environment with isolation, heating, and removal of the causative drug. In addition, support with hydration, albumin replacement and a hypercaloric diet should be provided. The main complications are sepsis (secondary to cutaneous infection) and severe ocular and genital lesions. Ocular lubricants are necessary, and the patient must be monitored by an ophthalmologist because eye sequelae are the main cause of disease morbidity.5 The use of corticosteroids is controversial. Other options for treatment include immunoglobulin and cyclosporin. However, the efficacy of these measures is still controversial.3
Yellow fever is an endemic disease in Brazil, and registered epidemic outbreaks signal the reemergence of the virus, as occurred in late 2017 in Jundiaí (SP). A large proportion of cities have low vaccination coverage and require mass vaccination of the local population.
The most effective method to prevent yellow fever is vaccination with the 17D sample. Currently, pregnant women, immunosuppressed patients, and people with a history of allergy to egg protein should not be vaccinated, due to the risk of developing type I allergic reaction (anaphylaxis).
We report a rare case of TEN triggered by yellow fever vaccination, given the importance of this serious adverse effect. Until now, the manual of the Brazilian Ministry of Health has made no mention of TEN as a possible adverse reaction to yellow fever vaccine.
Financial support: None.
Conflict of interest: None.