Dear Editor,
Chronic leg ulcers (LU) are those that do not heal in four to six weeks, remaining stagnant in the inflammatory phase.1 The influx of polymorphonuclear cells to the wound site, with release of lysosomal enzymes and oxygen free radicals, contributes to cell injury and disease maintenance, impairing healing.2
Uric acid (UA) has historically been associated with situations of increased oxidative stress, playing an important role in maintaining the inflammatory process on surfaces where there is tissue necrosis, and in triggering the inflammatory cascade.2
Fernandez et al.3 demonstrated that UA levels in the wound fluid of patients with LU are high and that its concentration is proportional to ulcer severity. The same authors also observed that xanthine oxidase, an enzyme that catalyzes the production of UA, is active in chronic wound secretions.2
After approval of the local Committee of Ethics in Research and signed consent, we studied 70 patients (mean age 63.3 ± 12.1 years, 60% women) to observe whether serum UA levels influence tissue healing and the presence of pain in venous LU.
Data collection was done in two visits, three months apart. During this time, all patients received standard treatment. During both visits, we recorded the ulcers' appearance, number and size. We also recorded the degree of pain using a visual analogue scale (VAS) ranging from 0 to 10, where 0 = no pain and 10 = worst possible pain. The ulcerated area was calculated by multiplying the measurements, in centimeters, of the two main axes of the wound. For patients with multiple wounds, the final area was calculated by summing the individual measurements. Presence of granulation tissue, fibrin, hyperkeratosis and necrosis was noted. UA was measured using the dry chemistry method, during the first visit, after 8 hours of fasting. Values up to 6.2 mg/dL for women and up to 8.5 mg/dL for men were considered normal.
The degree of healing was evaluated by comparing the number and area of the ulcers between the first and second visits.
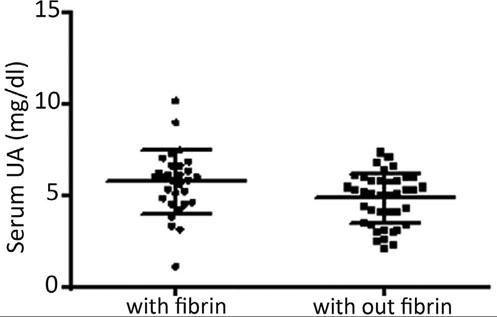
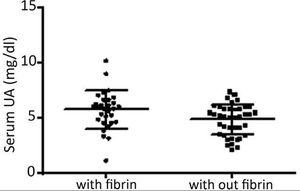
In this sample, 25.7% of the subjects were hyperuricemic. At the first visit, the median number of ulcers/patient was 1.0; the median area of ulcer/patient was 18 cm2, and the mean VAS pain was 3.0. Granulation tissue was present in 98.5%, fibrin in 42.8%, hyperkeratosis in 2.8%, and none had necrotic tissue. At this first visit, a correlation was found between the degree of pain and the level of UA (p = 0.02, Rho = 0.27, 95% CI = 0.03-0.47), but not between pain and the area or number of ulcers (p = ns). Figure 1 shows an association study of serum UA with ulcer characteristics.
Association study of serum uric acid (UA) with fibrin presence in leg ulcers (with fibrin, mean UA of 5.7 ± 1.7mg/dL; without fibrin, mean UA of 4.8 ± 1.3mg/dL; p = 0.01). No association was found with hyperkeratosis (p = 0.42). Presence of granulation and necrosis could not be associated with UA levels due to the small size of one of the samples
There was no correlation between serum UA and the differences (delta) in the number and size of ulcers (all p=ns). The difference in pain showed a correlation with UA (p = 0.01; Rho = 0.29; 95%CI = 0.05-0.50).
This study shows that uricemia is associated with pain and increased fibrin formation in leg ulcers, but it does not affect the ulcer's healing rate.
Pain is a common complaint in patients with LU, and it is one of the main contributors to loss of life quality in this context.1 In gout, pain is caused by the release of mediators that stimulate nociceptors during crystal phagocytosis.4 However, it has been shown that urate crystals alone, without leukocyte infiltration, can also cause pain.4 In animals, UA promotes dose-dependent pain that can be prevented by treatment with vanilloid receptor-1 blockers, demonstrating a neurogenic component in the genesis of this symptom.4
We have no explanations for the association of serum UA levels with pain in LU; however, it is possible to hypothesize that an individual with higher levels of UA in the blood may also have higher UA levels in the ulcerated wound.
The association of UA with presence of fibrin was also observed. The accumulation of fibrin in tissues results from fibrinogen escape through capillary pores enlarged by increased local venous pressure. Burnand et al.5 suggested that pericapillary fibrin affects the diffusion of oxygen and nutrients, favoring ischemia and impairing healing. Thus, it is possible to theorize that elevated levels of UA are associated not only with the presence of fibrin but also with oxygenation impairment and diminished healing. However, we have not been able to demonstrate this last hypothesis.
We conclude that UA levels are associated with pain severity and with the local formation of fibrin in LU, demonstrating that this radical is somehow involved in the pathophysiology of chronic ulcers.