Background: Brazil does not have a rosacea-specific quality of life questionnaire.
Objectives: translation into Brazilian Portuguese, development of cultural adaptation, and validation of the RosaQoL disease-specific questionnaire for rosacea of any subtype.
Methods: the recommended procedures for translation, cultural adaptation, and validation of an instrument were followed, and three interviews were conducted: baseline; seven to fourteen days after baseline; and at four to six months. The questionnaire was analyzed (with 95% confidence interval) for reliability by internal consistency (Cronbach’s alpha); test-retest reproducibility (intraclass correlation coefficient); responsiveness and validity.
Results: terms of the original questionnaire were replaced to guarantee cultural and semantic equivalence. Validity was demonstrated by expressive correlations between the RosaQoL domains and by significance in the Jonckheere-Terpstra test (p=0.05) between the scores of the RosaQoL domains and the participants’ self-perception in relation to the disease. Reliability was acceptable; alpha coefficient ranged from 0.923 to 0.916 in the first and second applications of the RosaQoL, respectively, and the Intraclass Correlation Coefficient (ICC) ranged from 0.671 to 0.863 in the seven- to fourteen-day period. Responsiveness, measured by grouping participants into three categories based on self-perception of rosacea (better, worse or unchanged), was found for the “better” response group (p=0.05).
Study limitations: small sample; limited variety of screening sources.
Conclusions: RosaQoL-BR (Brazil) was demonstrated as a reliable, valid and responsive questionnaire, with limitations, for individuals with any subtype of rosacea.
Rosacea is a chronic dermatological condition that affects the central region of the face (nose, malar region and forehead). Although its etiopathogenesis is not completely understood, dysregulation of the innate immune system, colonization by the skin’s commensal organisms, such as Demodex folliculorum and Helicobacter pylori, and neurovascular alterations are noted factors responsible for the appearance of the clinical aspects. Other possible triggers and aggravating factors are sun exposure, heat, spicy foods, alcohol abuse, emotional state, depression and migraine.1 According to the National Rosacea Society (NRS), rosacea classification comprises four subtypes, or recognized clinical forms, and one variant: I) erythrematotelangiectatic, II) papulopustular, III) phymatous, and IV) ocular (represented by blepharitis and conjunctivitis), and the variant, granulomatous rosacea. Each subtype is differentiated by the presence of primary and secondary characteristics, such as flushing, persistent erythema, telangiectasias, and outbreaks of inflammation with onset of papules and pustules. Such a condition can therefore greatly impair the affected person’s appearance and have a negative impact on their quality of life (QoL).2
In 2010, the worldwide prevalence of rosacea was 10% of the world population. Affected individuals are mostly fair skinned (mainly Caucasians with Celtic descent), women more often than men (3:1), and between 30 and 60 years of age; involvement of convex areas of the face is predominant.3 Most studies come from the United States and European countries. Regarding the epidemiology in Brazil, there is only one published study—it focused on the resident population in the south of the country and concluded that rosacea is most frequent in women between 40 and 50 years of age, predominantly in phototypes II and III, and the most common subtype is papulopustular. Knowledge and publications about rosacea are scarce in the Southern Hemisphere, especially in Brazil.4
Rosacea has a negative impact on QoL, increasing susceptibility to depression, social phobia, stress, and heightened perception of the disease. Unlike patients diagnosed with hypertension and diabetes, those with dermatological disorders have the perception that their illness is observed by all. Therefore, the psychosocial impact can be more severe and debilitating. A review of the literature showed that patients who experienced clinical improvement through treatment also showed improvement in psychological symptoms.5
The term QoL can be conceptualized through two definitions: one related to the subjective scope and another to the multidimensional scope. The subjective scope refers to an individual’s perception and expectations about their illness and its treatment. The multidimensional scope corresponds to a set of items related to mental health, physical and functional capacities, and social dimension, among others. Some researchers, for lack of consensus on the definition, consider the terms “health status” and “functional status” as synonyms for “QoL”, and several meanings are attributed to the economic, demographic, anthropological, bioethical, environmental, spiritual, and public health perspectives.6-9
There is another conceptualization of the term: QoL as a more general concept than Health Related QoL (HRQoL). QoL has a broader approach: it includes employment, family and friends, accounting for the influence of sociological factors, while omitting references to dysfunctions or disorders, which are aspects addressed in the HRQoL.6,8 Some authors believe that the introduction of QoL in the area of health probably occurred due to three factors: technological advances that provided better conditions for health recovery and prolongation of life; increased visibility of chronic diseases; and a change in the way the human being is viewed—historically as a biological organism, and in modern times as a social agent.8
A questionnaire is characterized by items, which can also be called questions. Several items are grouped according to the similarity of their concepts, creating domains, dimensions, subscales and even components that denote a specific aspect of QoL (social, physical, functional or psychological).10 Examples of domains are "functional capacity", which includes items that address the “mobility” and “self-care” aspects, and “emotional component", which includes items related to depression, anxiety and well-being.11-13
Studies have shown that clinical parameters of skin diseases are often poorly related to their impact on QoL and that most physicians underestimate this impact. Therefore, an instrument that measures and quantifies the relationship between rosacea and quality of life would be useful for physicians and researchers.3
Although there are several generic questionnaires that can be used to compare impacts on QoL between different diseases and populations, they may not be sensitive to the specificities of a disease.3 A disease-specific questionnaire is sufficiently sensitive to detect impacts on QoL and is also more sensitive to changes in the disease over time.3 Currently, there is no rosacea-specific questionnaire that has been translated, culturally adapted and validated for Brazilian Portuguese. The purpose of this study was to assess the feasibility of using this type of questionnaire. To that end, we used an instrument called RosaQoL, which was developed in the United States. The RosaQoL questionnaire was translated, culturally adapted and validated for Brazilian Portuguese, and its psychometric measures were analyzed.
MethodsThe study was approved by the Research Ethics Committee of the Federal University of São Paulo (CAAE: 45077015.8.0000.5505), and all participants signed the consent form.
The evaluated and included participants were those who sought the general screening branch of the São Paulo Hospital of the Federal University of São Paulo (HSP-UNIFESP), as well as screening at the General Ambulatory of Dermatology and at the Cosmiatry, Surgery and Oncology Unit (UNICCO) of the Department of Dermatology of the Federal University of São Paulo (UNIFESP), and who were later referred to the São Paulo Hospital.
All participants were evaluated by the investigator, based on the São Paulo Hospital’s electronic medical records and the information therein as it related to the inclusion and exclusion criteria. Information on the diagnosis of rosacea and its classification (subtype) was collected through the participants’ medical records. There was no clinical examination by a dermatologist at the time of inclusion in this study.
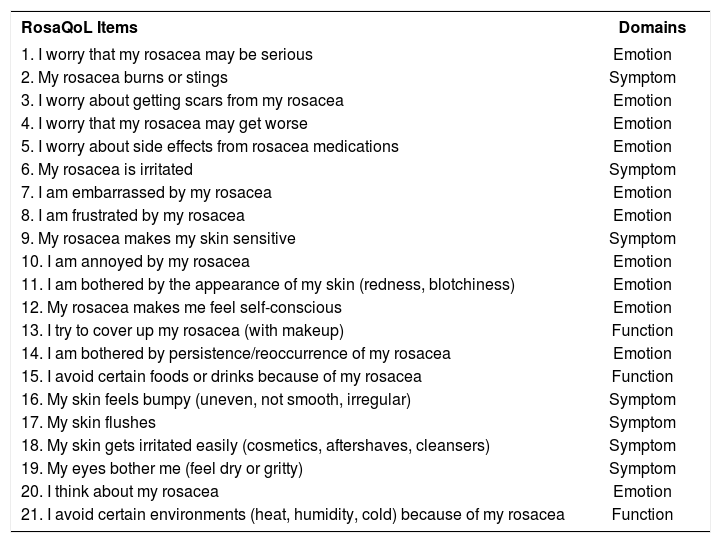
Participants were interviewed by the investigator ("face-to-face"), using both the RosaQoL and SF-36 questionnaires, in that order, in the first interview. RosaQoL is a disease-specific instrument and can be answered quickly. It consists of 21 items related to rosacea, subdivided into three domains (Emotion, Symptom and Function); five items in a “General Questions” domain; and a final question that was answered only during the third visit, which is: “How is your rosacea compared to the last time you filled out this questionnaire?” (Chart 1).3 The SF-36 generic questionnaire was used as a gold standard, i.e., as a complement to the Health Quality of Life (HRQoL) assessment, through the correlation between its scores and those of the RosaQoL version.
RosaQoL items and domains
| RosaQoL Items | Domains |
|---|---|
| 1. I worry that my rosacea may be serious | Emotion |
| 2. My rosacea burns or stings | Symptom |
| 3. I worry about getting scars from my rosacea | Emotion |
| 4. I worry that my rosacea may get worse | Emotion |
| 5. I worry about side effects from rosacea medications | Emotion |
| 6. My rosacea is irritated | Symptom |
| 7. I am embarrassed by my rosacea | Emotion |
| 8. I am frustrated by my rosacea | Emotion |
| 9. My rosacea makes my skin sensitive | Symptom |
| 10. I am annoyed by my rosacea | Emotion |
| 11. I am bothered by the appearance of my skin (redness, blotchiness) | Emotion |
| 12. My rosacea makes me feel self-conscious | Emotion |
| 13. I try to cover up my rosacea (with makeup) | Function |
| 14. I am bothered by persistence/reoccurrence of my rosacea | Emotion |
| 15. I avoid certain foods or drinks because of my rosacea | Function |
| 16. My skin feels bumpy (uneven, not smooth, irregular) | Symptom |
| 17. My skin flushes | Symptom |
| 18. My skin gets irritated easily (cosmetics, aftershaves, cleansers) | Symptom |
| 19. My eyes bother me (feel dry or gritty) | Symptom |
| 20. I think about my rosacea | Emotion |
| 21. I avoid certain environments (heat, humidity, cold) because of my rosacea | Function |
The SF-36 is an easy-to-use multidimensional indicator and consists of 36 items grouped into eight dimensions: functional capacity (ten items); limitations caused by physical health problems (four items); emotional aspects (three items); mental health (five items); pain (two items); vitality (i.e., energy/fatigue, four items); general condition (five items); and a comparative evaluation question regarding one’s health status currently and one year before. Most of the questions refer to the last four weeks.10,14,15 A license to use the SF-36 was requested (license number: QM038721).
After a minimum of seven and a maximum of fourteen days after the first interview, the same participants were invited to attend the second interview, in which only the RosaQoL was applied. Finally, participants of the second interview were invited to a third interview, four to six months later, for the application of only the RosaQoL. After the third interview, participants were referred for rosacea treatment at the São Paulo Hospital outpatient clinic. All questions were answered in the first interview. Demographic questions were omitted in the second and third interviews. In the third interview, patients were questioned about self-perception of their rosacea: “worse", “no change” or “better”.
After the three interviews, a database was built. Data from participants who did not return for the second or third interview or who returned after the maximum 14-day interval were not included in the data analysis for the assessment of test-retest reproducibility and responsiveness of RosaQoL.
Data collection took place between January and December 2016.
The inclusion criteria were:
- •
men and women between the ages of 18 and 75;
- •
men and women with rosacea of any type and severity;
- •
ability to understand and agree to study conditions and to sign the ICF.
The exclusion criteria were:
- •
patients who received a new drug under investigation in the past 30 days;
- •
patients with a history of psychological illness or conditions that could interfere with the ability to understand the conditions of the study.
The collected variables in the first interview were related to clinical and demographic characteristics: age, gender, marital status, race, questions 1 and 2 of the “General Questions” of the RosaQoL questionnaire, and duration of rosacea.
All analyses were performed with the software (SPSS, Version 21.0 for Windows, SPSS Inc, Chicago). Non-parametric tests were performed, because the data did not present a normal distribution.
The score of each RosaQoL questionnaire was obtained by averaging the responses to the 21 items. For example, the emotion score is a result of the average of all responses to the items relating to the emotion domain. The answers to the items were “never", “rarely", “sometimes", “often” and “always”. Responses were recorded on the scale of 1 (never) to 5 (always). The SF-36 score was also calculated through some specificities of this questionnaire (with scores ranging from 0 to 100), so that it was possible to compare the scores of RosaQoL with those of the SF-36. It is important to highlight that the higher the RosaQoL score, the worse one’s quality of life, and the lower the score, the better one’s quality of life. The opposite occurs with the SF-36: higher scores indicate higher QoL, and lower scores indicate lower QoL.3,15
The questionnaire was translated into Brazilian Portuguese by two independent, qualified bilingual translators who were aware of the research objective. The researcher and three bilingual dermatologists then analyzed the translations to unify them into a Portuguese version. This version was converted into English by two experts and native speakers of the language, who did not know the purpose of the research. The researcher and translators converted the English versions into a single version. A committee, composed of three bilingual dermatologists and the researcher, reviewed the final Portuguese version and the final English version, comparing them in order to avoid semantic and grammatical discrepancies between the two languages. A pilot study was conducted with a group of 15 participants who were rosacea patients from the General Ambulatory of the Department of Dermatology - UNIFESP and had signed the consent form. The purpose was to evaluate the comprehensibility of the items of the questionnaire, which was applied by the researcher of the study.
The validity of an instrument can be analyzed by a number of methods, but there is no specific coefficient that reflects this property. The validity of the Portuguese language version of the RosaQoL was measured through the following analyses:
- •
correlation between domains and total score of RosaQoL;
- •
correlation between the domains and total score of RosaQoL and domains of the generic questionnaire SF-36;
- •
correlation between total score/score of each domain of the Portuguese version of the RosaQoL (RosaQoL-BR) and variables related to QoL, such as questions 1, 2 and 3;
- •
comparison between QoL scores and demographic and clinical characteristics (marital status, gender, duration of rosacea, and race);
- •
correlation between age and domain scores and total score of the RosaQoL questionnaire.
The reliability of the RosaQoL was constructed and analyzed from Cronbach’s Alpha Coefficient and Intraclass Correlation Coefficient (internal consistency analysis and test-retest reproducibility, respectively). The set of 21 items and the items corresponding to each domain were analyzed for each participant’s visits corresponding to moments zero and two (seven to fourteen days later). A minimum of seven and a maximum of fourteen days were allowed between these visits. The participants were grouped according to the time between the baseline visit and the second interview (exactly seven days and more than seven days).16
The responsiveness of the questionnaire was analyzed based on the calculations referring to the first interview (baseline visit) and the third (four to six months after the baseline visit). The Wilcoxon test was performed, since the data were non-parametric. The participants were grouped based on the answer given to the following question, performed only in the third interview: “How is your rosacea compared to the last time you filled out this questionnaire?” The possible responses to this question were: “ Worse", “No change” and “Better”.
For statistical analysis, we characterized the variables present in the study and defined the types of variables. Thus, we performed a descriptive statistical analysis, obtaining values of mean, median, percentiles, standard deviation, minimum value, maximum value, confidence interval and kurtosis. Tests for comparison of means and variances between groups, linear regression and correlation were performed to construct an analysis of psychometric measures. For validity, we used Jonckheere-Terpstra (non-parametric test) and Spearman correlation (non-parametric test). For reliability, Intraclass Correlation Coefficient (test-retest reproducibility) and Cronbach’s alpha tests (internal consistency) were used. Finally, Wilcoxon’s test (non-parametric test) was used for three different groups: improvement, no change, and worsening of rosacea, comparing between the patients’ reports in the first and third interviews. It is important to point out that all the statistical analyses were based on a 95% confidence interval. Categorical variables were expressed as absolute frequency (N) and relative frequency.
ResultsIn the first interview, the RosaQoL questionnaire and the SF-36 questionnaire were answered by 60 participants. Only 48 respondents answered RosaQoL in the second interview, and 38 in the third interview. The participants took an average of 8 ±2 minutes to answer the questionnaire.
After the committee’s evaluation and the pilot test, some expressions and words were substituted to guarantee cultural, idiomatic, semantic and conceptual equivalence.
Among the participants who performed the first interview, 49 were women and 11 were men. Of these participants, the mean age was 48.43 years; 31.7% were single or never married, 51.7% were married or living with partner, and 16.7% had another civil status; 87.93% were white, and 12.07% were brown or mulatto.
Psychometric measures were evaluated (validity, responsiveness and reliability).
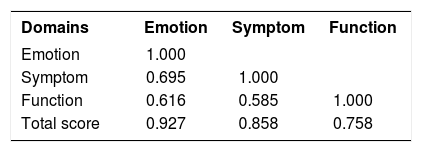
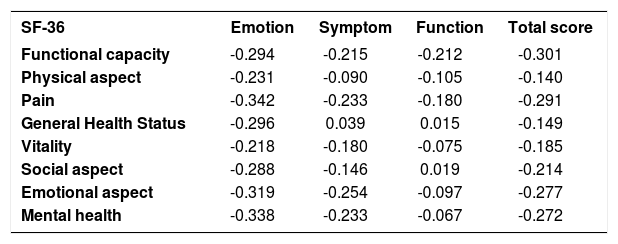
ValidityAfter the univariate analysis (descriptive statistics) of the scores of all domains and the total score of the RosaQoL-BR (Brazil) from the first and second interviews, we analyzed the correlation between the RosaQoL-BR domains. That analysis resulted in more expressive correlations between all components (correlation was high and moderate) and between all of the RosaQoL-BR and SF-36 domains, the correlations of which were negative. The most expressive but weak correlation was between SF-36 pain and RosaQoL-BR emotion. This data is shown in the chart 3 and 4.
Correlation between RosaQoL-BR domains and SF-36 domains
| SF-36 | Emotion | Symptom | Function | Total score |
|---|---|---|---|---|
| Functional capacity | -0.294 | -0.215 | -0.212 | -0.301 |
| Physical aspect | -0.231 | -0.090 | -0.105 | -0.140 |
| Pain | -0.342 | -0.233 | -0.180 | -0.291 |
| General Health Status | -0.296 | 0.039 | 0.015 | -0.149 |
| Vitality | -0.218 | -0.180 | -0.075 | -0.185 |
| Social aspect | -0.288 | -0.146 | 0.019 | -0.214 |
| Emotional aspect | -0.319 | -0.254 | -0.097 | -0.277 |
| Mental health | -0.338 | -0.233 | -0.067 | -0.272 |
In addition to the correlations, analyses of variance and comparisons were evaluated. Regarding the self-perception of rosacea severity during the four weeks prior to the first interview’s questionnaire, we found that 23.33% of respondents answered “bad” and 21.67% answered “good”. Of all 60 participants, 25% had rosacea for 2 to 5 years; 25% had the condition for 5 to 10 years; and another 25% had rosacea for over 10 years. In the self-assessment of rosacea’s influence on QoL, 23% answered “a lot” and 20% answered “none”.
In the linear regression analysis, the scores for all domains and total score of the RosaQoL-BR questionnaire represented the dependent or response variable and the participants’ age represented the independent or explanatory variable. We found that the independent variable “age” was present in the model and was independently associated with all domains and the total score of the RosaQoL-BR questionnaire.
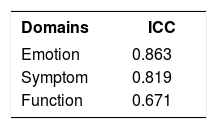
ReliabilityItems from the three domains showed internal consistency according to Cronbach’s alpha coefficient, which ranged from 0.923 to 0.916 (in the first and second applications of RosaQoL-BR, respectively; Chart 5). After suppression of an item, there was no significant increase in the coefficients. The items also showed reproducibility through the intraclass correlation coefficient (ICC), which ranged from 0.671 to 0.883 for the seven- to fourteen-day interval (Chart 6).
Participants were divided into three categories (better, worse or unchanged), based on their self-perception. Comparing the third interview with the first, seventeen participants reported improvement and six reported worsening. For participants who reported improvement in rosacea, we observed an improvement in the questionnaire scores (with a statistically significant difference, Wilcoxon test - p <0.05), and for participants who reported no change, there was no difference in the scores (no statistically significant difference was found, Wilcoxon test - p> 0.05).
No significant difference was found in those who reported worsening.
DiscussionThis study represented the translation, cultural adaptation and validation of the RosaQoL questionnaire, which is specific for rosacea. We followed the procedures recommended in the international literature, including psychometric analysis and assessments of reliability, responsiveness and validity.17-19
Reliability was demonstrated through two methods: internal consistency according to the Cronbach coefficient and reproducibility according to the intraclass correlation coefficient. Thus, RosaQoL-BR is guaranteed to produce consistent results for patients with rosacea, under similar conditions and without random errors.
Responsiveness was demonstrated by decreased scores for those participants who reported improvement in rosacea four to six months after the first interview, and by unchanged scores for those who reported their rosacea as unchanged or worsening. The scores for participants with worsening rosacea showed no statistical significance (Wilcoxon test, p> 0.05), probably because only six subjects provided this response, representing a small sample. Therefore, further studies should be performed with larger samples to see whether there is an increase in the score for those participants who report worsening of rosacea.
This study confirmed the validity of the RosaQoL-BR, showing correlation between the “Emotion", “Symptom” and “Function” domains’ scores and the participants’ self-perception regarding the severity of rosacea. In addition, the moderate to very strong correlations between the RosaQoL-BR domains, as well as between the domain scores and the total score, proved the unity of the questionnaire. Among the domain scores, excepting the total score, the highest correlation was obtained between the “Emotion” and “Symptom” domains, which confirms the body-image and appearance concerns of individuals with rosacea.
The validity of the RosaQoL-BR was also verified through its comparison to the domains of the SF-36 questionnaire. The correlation was negative—in the SF-36, the lower the score, the greater rosacea’s impact on QoL, while in the RosaQoL-BR, the higher the score, the greater rosacea’s impact on QoL. There was a very weak to weak correlation in relation to the SF-36 domains “Social aspect” and “Mental health", which can be explained by the fact the questionnaire addresses items of emotional aspect strongly related to anxiety and depression, whereas the RosaQoL-BR consists of items specific to rosacea patients.
It was not possible to conclude whether there was a correlation between age and the scores of the questionnaire, since the correlation coefficient (r) remained between 0.047 and 0.254 for all domains and the total score of the RosaQoL-BR questionnaire. In the linear regression analysis, there was no trend of linearity between the points, probably due to the sample size being too small to detect significant differences in the different age groups. In addition, the sample was predominantly elderly people.
In the comparison between the domains, the total score of the questionnaire and the categories of duration of rosacea, there was no significant difference; i.e., the duration of the disease did not influence the scores. We need to mention that, since rosacea duration is a categorized variable, the values ought to be distributed in mutually exclusive categories. In other words, it should not be possible for an individual to give the same answer via two different response options. However, as discussed previously, the answer of “2 years” is available as a response option in two different categories: “1-2 years” and “2-5 years."
Regarding the gender variable, there was a significant difference in the total score and in the “Emotion” and “Symptom” domains of the RosaQoL-BR questionnaire. The “Symptom” domain and the total score showed that rosacea had a greater impact on the quality of life of females. The “Emotion” domain showed, albeit subtly, a greater impact for males. The greater impact on the QoL of females corroborates the results of Morgan et al., 199720 and Kellett et al., 1999,21 which showed that women present greater QoL impairment due to the predominantly female characteristic of perceiving their image in a distorted way (body dysmorphic disorder).
The race variable was found to have no impact on the quality of life of the study participants.
The study results are limited by some factors. One of them is the sampling method, which involved a passive search for rosacea patients. It is probable that, due to this type of search, the sample did not represent the distribution found in other studies, in relation to age, gender, marital status, duration of illness, and race. Another factor is that the sample was limited, since it was obtained from a narrow variety of sources.
In addition, the instrument’s responsiveness was validated only in patients who reported improvement in rosacea four to six months after the first interview. This may limit the use of the instrument in research studies where participants have rosacea. Despite the limitations, RosaQoL-BR allows the measurement of the QoL of individuals with rosacea and can feasibly be used in clinical practice or in research. Most evaluations for therapeutic intervention are established only by clinical parameters. However, QoL is another important parameter, capable of increasing the empathy between the physician and the patient, resulting in greater adherence to the treatment.3
ConclusionRosaQoL-BR assists in a personalized treatment, allowing individuals to be monitored over time. With this questionnaire, the impact of the disease on QOL materializes, contradicting the perception that rosacea is a cosmetic issue. It can be used in the research and development of new therapies and as complementary to secondary or co-primary goals.3
It is worth highlighting the relevance of future studies with a larger sample coming from different sources of participant screening, such as different cities, diverse regions of the country, and a wider distribution of age groups. It is important that these studies include variables such as rosacea subtypes, rather than disease duration or seasonal effects, and the tendency toward psychological disorders (e.g. depression and anxiety); they should also explore the different ways of administering the questionnaire (self-administration, face-to-face or telephone interview).
Financial support: Project funded by FAPESP, number 2015/18924-0.
Conflict of interest: None.