Melanoma is a malignant neoplasia that shows high mortality when diagnosed in advanced stages. Early identification of high-risk patients for the development of melanoma metastases is the main strategy to reduce mortality.
Objective:To assess the influence of eight epidemiological and histopathologic features on the development of metastases in patients diagnosed with primary cutaneous melanoma.
Methods:Our historical cohort comprised patients with invasive primary cutaneous melanoma seen between 1995 and 2012 at a public university hospital and a private oncologic surgery institution in Southeastern Brazil. The following variables were analyzed: gender, age, family history of melanoma, site of the primary tumor, clinical and histologic subtype, Breslow thickness, histologic ulceration and the mitotic index. Kaplan-Meier univariate test and multivariate Cox proportional hazard analysis were used to assess factors associated with disease-free survival.
Results:Five hundred and fourteen patients were enrolled. The univariate analysis identified the following significant risk factors: gender, age, site of the tumor, clinical and histologic subtype, Breslow thickness, histologic ulceration and mitotic index. Multivariate analysis included 244 patients and detected four significant prognostic factors: male gender, nodular clinical and histologic subtype, Breslow thickness > 4mm, and histologic ulceration. The mitotic index was not included in this analysis.
Study limitations:Small number of patients in multivariate analysis.
Conclusions:The following prognostic factors to the development of melanoma metastasis were identified in the study: male gender, nodular histologic subtype, Breslow thickness > 4mm and ulceration.
Melanoma is an aggressive tumor with high mortality when diagnosed in advanced stages. Its incidence is increasing drastically all over the world, as well as its cases of death.1,2 Despite promising therapeutic advances, treatments capable of significantly prolonging the survival of patients with metastasis have not yet been developed. For this reason, the main strategy to reduce mortality is based on the early identification of the primary tumor and of the patients at risk for metastatic disease.3
Different risk factors for the development of cutaneous melanoma metastases were studied extensively. Clinical and epidemiological aspects such as age, gender and site of the tumor are associated to disease progression.4 Certain histopathologic, immunologic, genetic and molecular characteristics were also implicated in the development of melanoma metastasis.1,5
Due to the alarming increase in the incidence of melanoma and to the high mortality rates from metastatic disease, the data of patients seen with primary melanoma in the department of dermatology of a university hospital and a private surgical oncology practice between 1995 and 2012 were analyzed with the aim of identifying prognostic factors for the development of metastasis in melanoma.
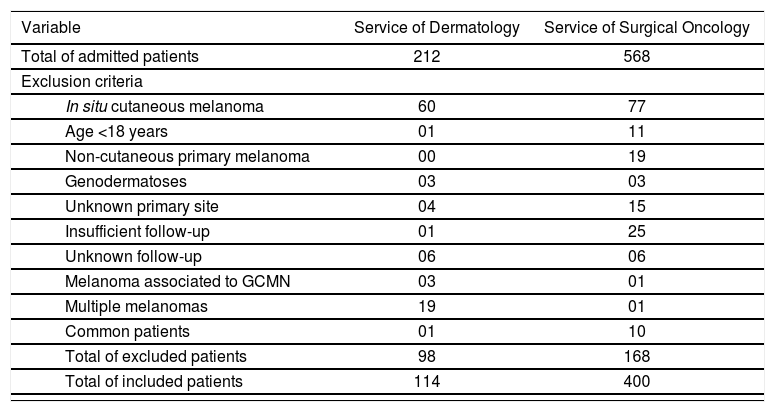
MethodsStudy PopulationThis is a historical cohort, with data collection from patients’ files. Between January 1995 and January 2012, 212 patients with suspicion of melanoma were admitted to the department of dermatology of the Hospital das Clínicas, Universidade Federal de Minas Gerais (HC-UFMG) and 568 patients to the private surgical oncology practice ONCAD (Oncologia Cirúrgica e Cirurgia do Aparelho Digestivo), both in the Southeastern region of Brazil. Of the total of 780 patients, 266 individuals were initially excluded: 137 with melanoma in situ, 12 younger than 18 years of age, 19 for having a non-cutaneous primary melanoma, 6 with the diagnosis of genodermatoses, 19 with unknown primary tumor, 26 with a follow-up shorter than 1 month, 12 with indeterminate follow-up, 4 with melanoma associated to giant congenital melanocytic nevus, 20 with multiple melanomas and 11 patients being followed in both services (table 1). Therefore, 514 patients were included in the study.
Patients included in and excluded from the final statistical analysis (1995-2012)
| Variable | Service of Dermatology | Service of Surgical Oncology | |
|---|---|---|---|
| Total of admitted patients | 212 | 568 | |
| Exclusion criteria | |||
| In situ cutaneous melanoma | 60 | 77 | |
| Age <18 years | 01 | 11 | |
| Non-cutaneous primary melanoma | 00 | 19 | |
| Genodermatoses | 03 | 03 | |
| Unknown primary site | 04 | 15 | |
| Insufficient follow-up | 01 | 25 | |
| Unknown follow-up | 06 | 06 | |
| Melanoma associated to GCMN | 03 | 01 | |
| Multiple melanomas | 19 | 01 | |
| Common patients | 01 | 10 | |
| Total of excluded patients | 98 | 168 | |
| Total of included patients | 114 | 400 | |
GCMN: Giant congenital melanocytic nevus
The patients selected for the statistical analysis fulfilled the following inclusion criteria: diagnosis of invasive primary cutaneous melanoma confirmed by histopathology, age equal to or above 18 years and a minimum follow-up time of 1 month.
Patient follow-up in each service was conducted according to well-established protocols in the literature. Clinical evaluation was trimonthly in the first year after the diagnosis, four to six-monthly in the second year and annually thereafter. Follow-up interval was reduced according to the presence of risk factors for tumor spread. Follow-up included physical examination, chest X-ray and levels of serum lactic dehydrogenase, with the goal of detecting recurrences and local or distant metastases. In the cases with risk for spread, other imaging studies were requested, according to the affected area. Patients classified as stage IB or II were referred for sentinel lymph node biopsy.
The project was approved by the Committee of Research Ethics of the institution.
The patients that were still being seen at the services during the time of data collection signed a consent form.
Anatomopathological examinationThe diagnosis of cutaneous melanoma was performed by anatomopathological examination. At the service of dermatology, only one experienced dermatopathologist was responsible for the analysis of the cases. At the service of surgical oncology, the diagnosis was obtained by the validation or review of the reports by a pathologist with a wide experience in melanocytic lesions.
Follow-upThe time for the follow-up of patients was defined as the interval between the date of diagnosis of the primary cutaneous melanoma (time zero) and the date of the last consultation or the date of the diagnosis of melanoma metastasis, in the cases where the disease spread (date of outcome). Data from patients’ files with a minimum follow-up of one month were included.
Statistical analysisDisease free survival was calculated considering the period between the diagnosis of primary cutaneous melanoma and the diagnosis of metastasis or the date of the last consultation. Survival analysis was calculated by univariate analysis, with the Kaplan-Meier curves, log-rank test and the multivariate Cox proportional hazard analysis to obtain the risk factors for the occurrence of metastasis of the 514 patients included in the study. The variables with p<0.25 in the multivariate analysis were submitted to analysis by the Cox model. The value of p<0.05 was considered statistically significant. Student t test was utilized to compare age means and Mann-Whitney test for the follow-up period. Schoenfeld residuals test was applied to the analyses to evaluate model adequacy and considered p>0.05 as adequate. Statistical analyses were performed using software R.
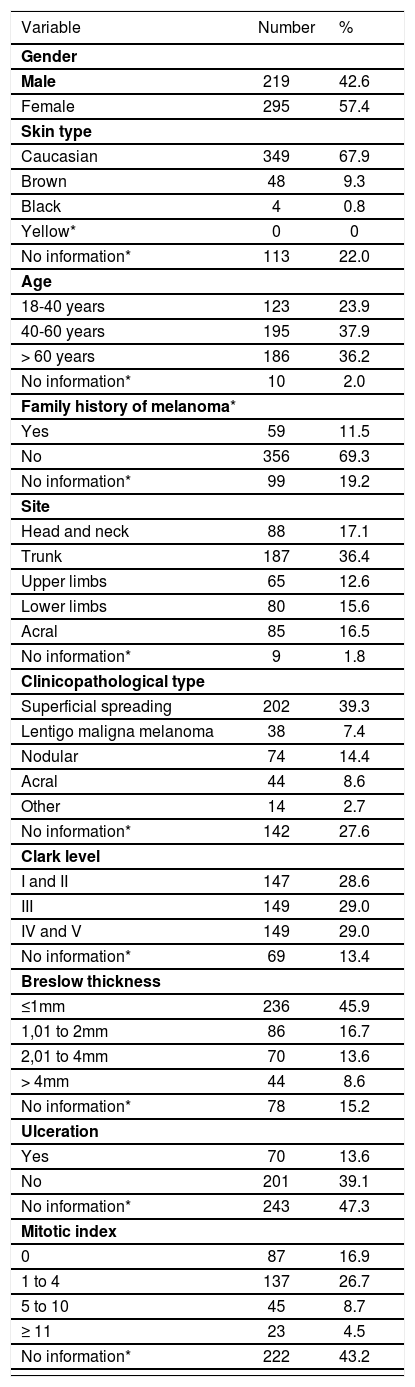
ResultsAnalysis of the characteristics of primary cutaneous melanomaAccording to clinical and epidemiological characteristics, most were females (57.4%), Caucasian (67.9%), older than 40 years (74.1%) and had no family history of melanoma (69.3%). Regarding the site, most tumors were located on the limbs (53.5%). Regarding the histologic features, there was a predominance of the superficial spreading histologic type (39.3%) and thin tumors (≤1mm) (45.9%). The distribution of the tumors between the different levels of Clark was similar (28.6 to 29%). Of the total of 514 patients, 271 (52.7%) had information of ulceration on the anatomopathological examination, with a predominance of non-ulcerated melanomas (39.1%). Among the 292 (56.8%) that had information of the presence of mitoses, most (39.9%) had at least 1 mitosis/mm2 (table 2).
Clinical, epidemiologic and histopathologic features of the patients seen at the services of dermatology and surgical oncology (1995-2012)
| Variable | Number | % |
|---|---|---|
| Gender | ||
| Male | 219 | 42.6 |
| Female | 295 | 57.4 |
| Skin type | ||
| Caucasian | 349 | 67.9 |
| Brown | 48 | 9.3 |
| Black | 4 | 0.8 |
| Yellow* | 0 | 0 |
| No information* | 113 | 22.0 |
| Age | ||
| 18-40 years | 123 | 23.9 |
| 40-60 years | 195 | 37.9 |
| > 60 years | 186 | 36.2 |
| No information* | 10 | 2.0 |
| Family history of melanoma* | ||
| Yes | 59 | 11.5 |
| No | 356 | 69.3 |
| No information* | 99 | 19.2 |
| Site | ||
| Head and neck | 88 | 17.1 |
| Trunk | 187 | 36.4 |
| Upper limbs | 65 | 12.6 |
| Lower limbs | 80 | 15.6 |
| Acral | 85 | 16.5 |
| No information* | 9 | 1.8 |
| Clinicopathological type | ||
| Superficial spreading | 202 | 39.3 |
| Lentigo maligna melanoma | 38 | 7.4 |
| Nodular | 74 | 14.4 |
| Acral | 44 | 8.6 |
| Other | 14 | 2.7 |
| No information* | 142 | 27.6 |
| Clark level | ||
| I and II | 147 | 28.6 |
| III | 149 | 29.0 |
| IV and V | 149 | 29.0 |
| No information* | 69 | 13.4 |
| Breslow thickness | ||
| ≤1mm | 236 | 45.9 |
| 1,01 to 2mm | 86 | 16.7 |
| 2,01 to 4mm | 70 | 13.6 |
| > 4mm | 44 | 8.6 |
| No information* | 78 | 15.2 |
| Ulceration | ||
| Yes | 70 | 13.6 |
| No | 201 | 39.1 |
| No information* | 243 | 47.3 |
| Mitotic index | ||
| 0 | 87 | 16.9 |
| 1 to 4 | 137 | 26.7 |
| 5 to 10 | 45 | 8.7 |
| ≥ 11 | 23 | 4.5 |
| No information* | 222 | 43.2 |
There was loss of data regarding the following variables: skin color (22%), age (2%), family history of melanoma (19.2%), site of the primary tumor (1.8%), clinicopathological subtype (27.6%), Clark level (13.4%), Breslow thickness (15.2%), ulceration (47.3%) and mitotic index (43.2%) (table 2).
Analysis of the characteristics of the patients with metastasisOf the total of 514 patients, 135 (26.3%) had the diagnosis of metastasis during follow-up: 14 (2.7%) seen at the department of dermatology and 121 (23.6%) seen at the service of surgical oncology. Two hundred and ninety-two metastases of the 135 patients were diagnosed in different sites and, in 7 of those, the site was not specified. Regional metastases corresponded to 36.3% of the cases, local corresponded to 19.5% and distant metastases were responsible for 44.2% of the total number of cases.
Among patients with melanoma metastasis, the mean age was 53 years (±17 years) and the median was 51 years. For those without metastasis, the mean age was 54 years (±17 years) and the median 53 years. There was no statistically significant difference between the groups (p=0.697). The median duration of the cohort’s follow-up was 24.7 months (ranging from 1 to 374). The median duration of follow-up for patients with metastasis was 12.2 months, significantly lower than those censored, which was 30.4 months (p<0.001).
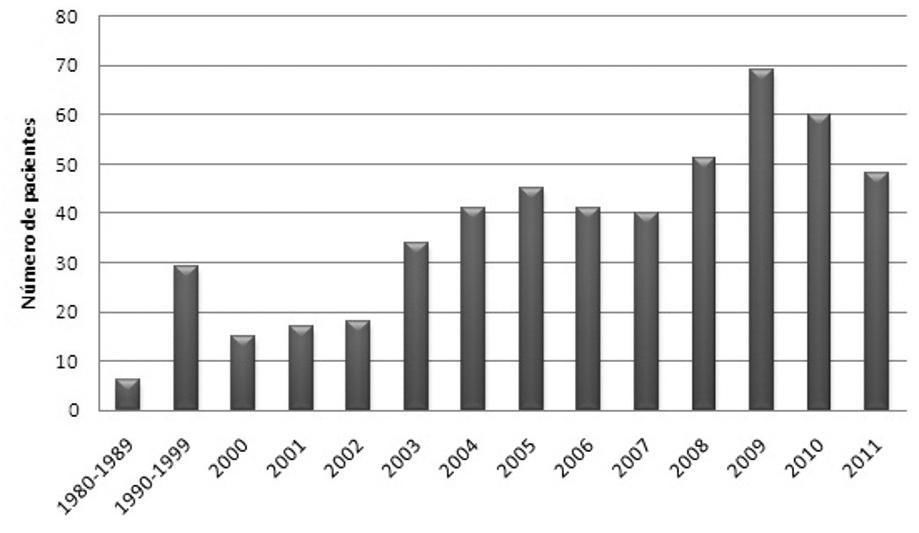
Of the 514 patients, 447 (87%) were diagnosed with the disease in the previous 10 years (Figure 1).
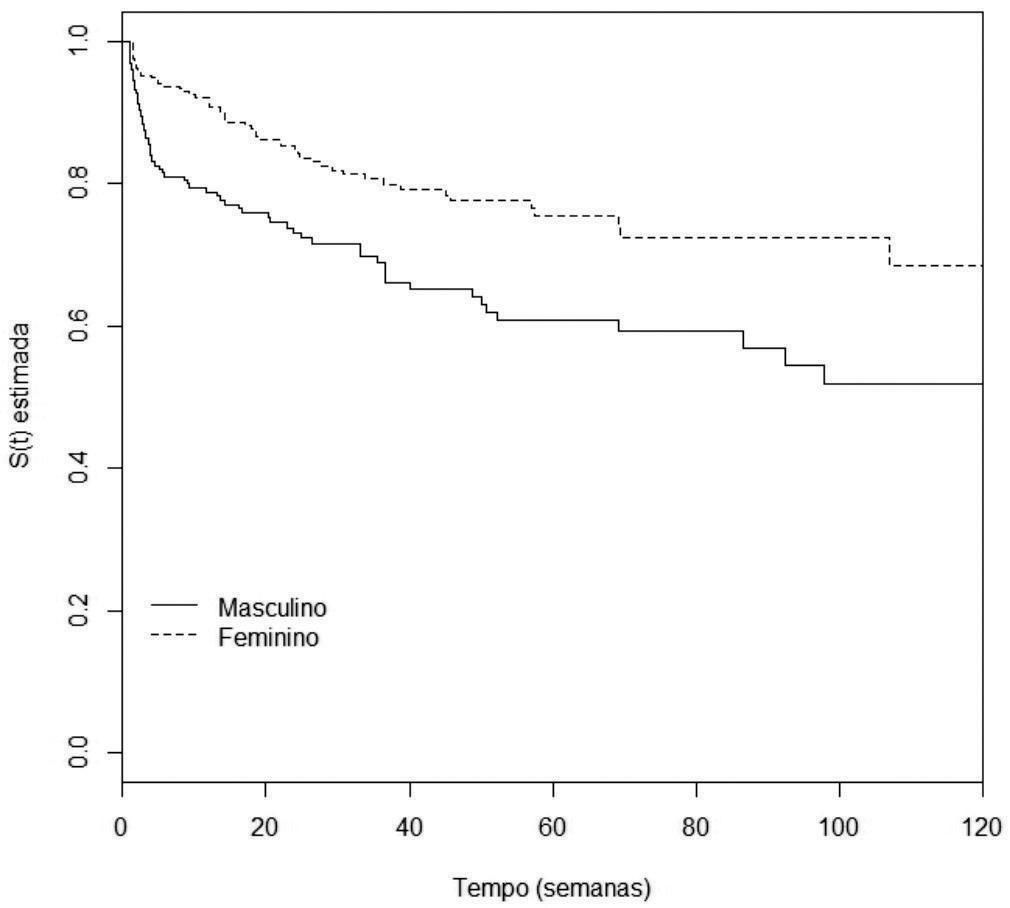
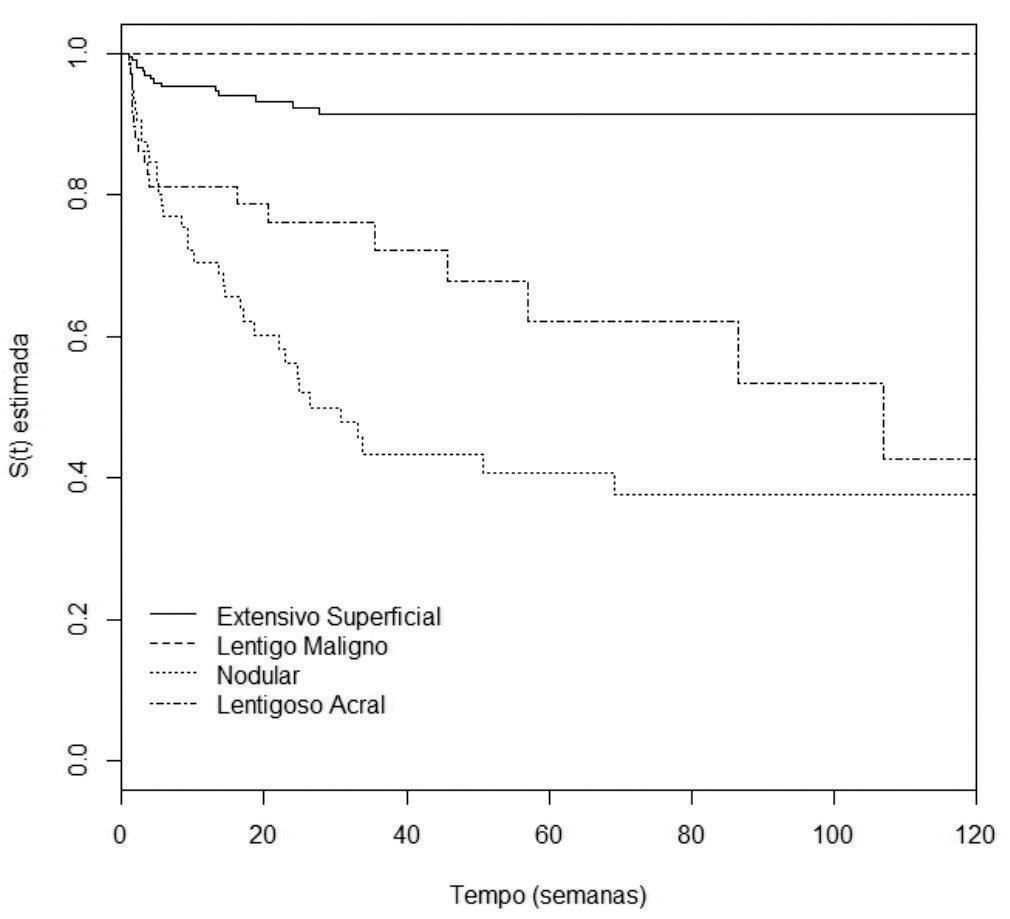
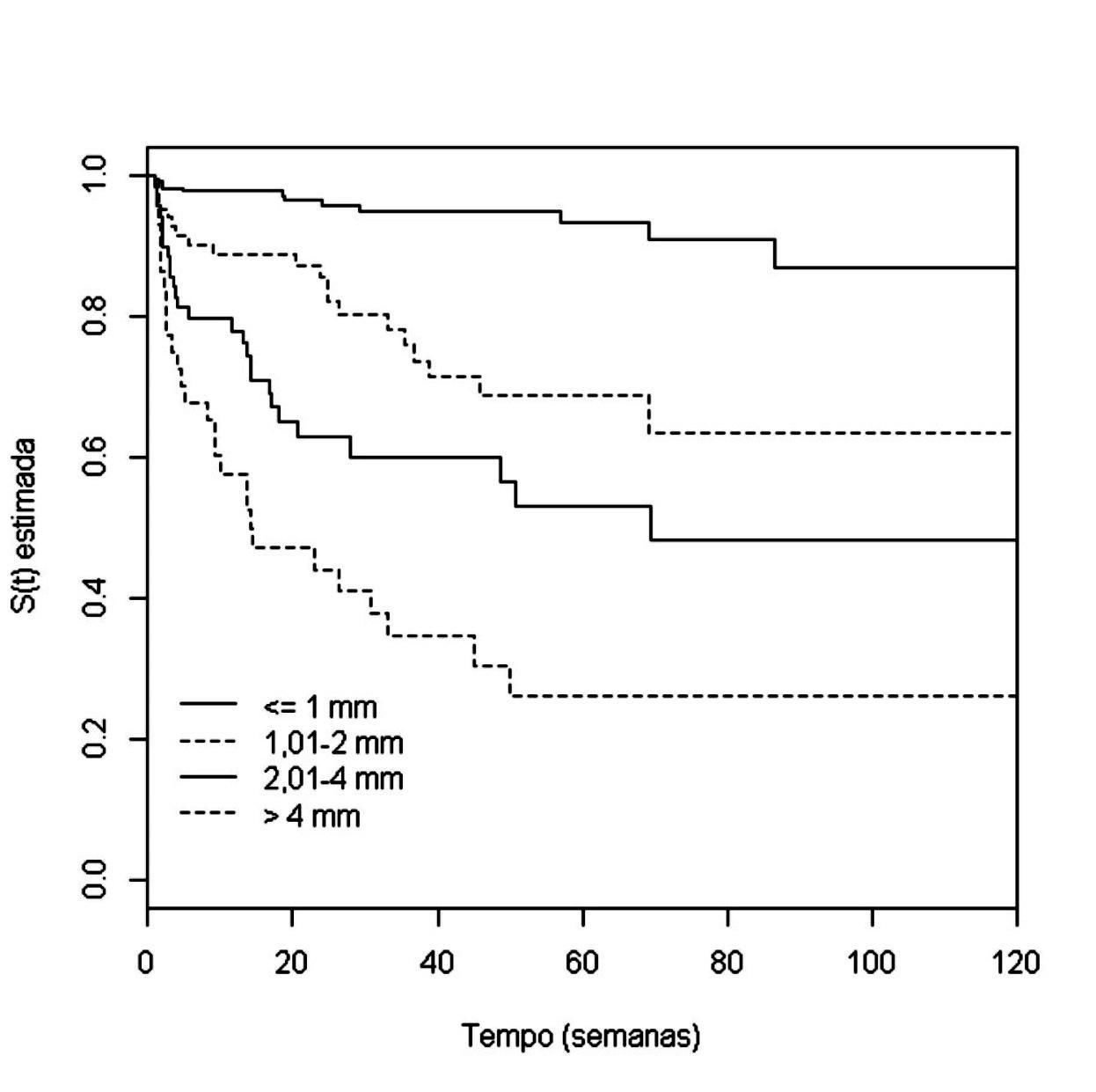
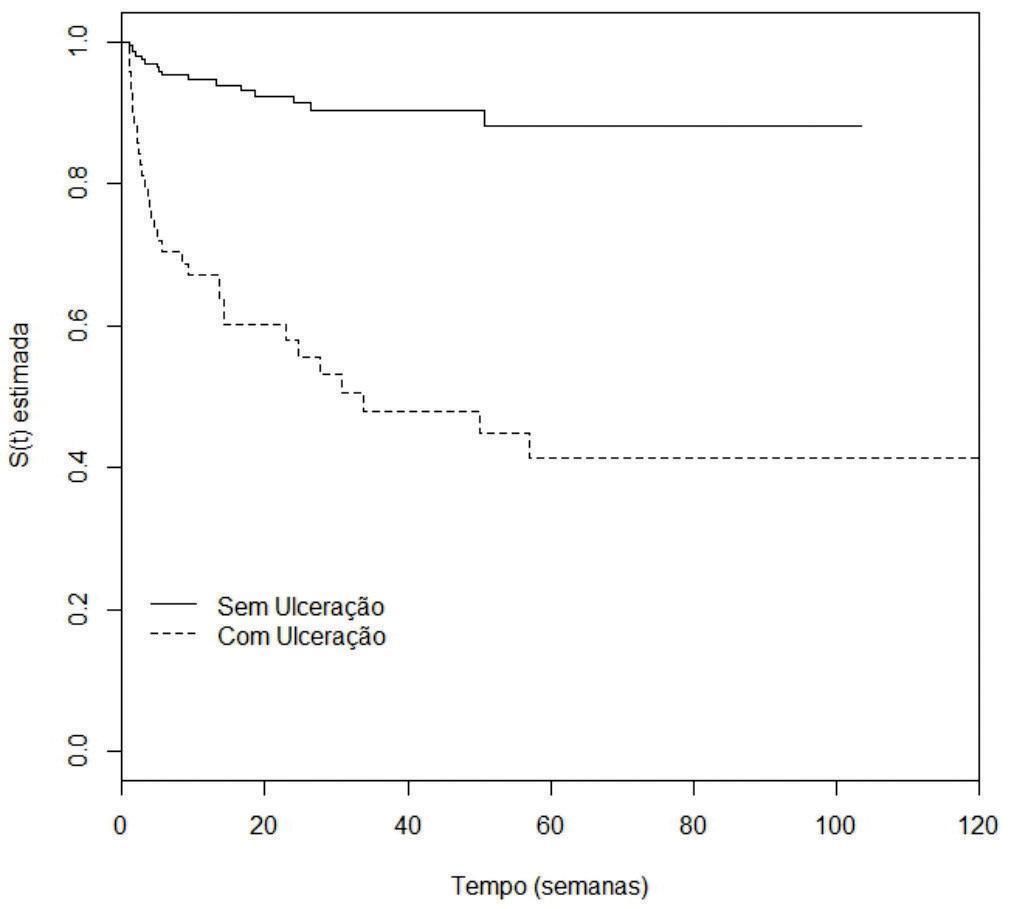
Analysis of disease-free survivalAccording to the univariate analysis by the Kaplan-Meier method and the log-rank test, the following variables were considered of risk for the occurrence of metastasis: male gender (p=0.0007), nodular and acral clinicopathological types (p<0.0001), Breslow thickness >1mm (p<0.0001), ulceration (p<0.0001) (Figures 2 to 5), age >60 years (p=0.0566), acral location of the primary tumor (p=0.0054) and mitotic index (p<0.0001). Family history was not a significant variable (p=0.985).
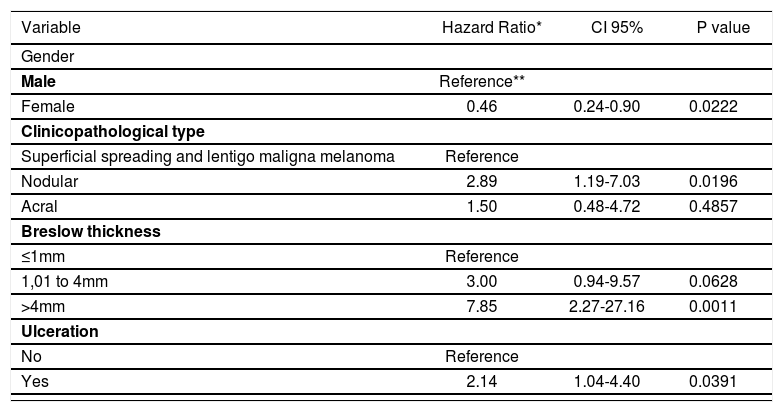
The multivariate analysis was conducted using the Cox model. It was not possible to include the mitotic index, for there were no patients with metastasis that did not have mitosis on the anatomopathological examination. The same occurred with the clinicopathological type lentigo maligna melanoma. For this reason, we opted to group it with the superficial spreading type, that presented the lowest melanoma metastasis risk when compared to the nodular and acral types. The variable of family history of the disease was not included because it did not reach statistical significance with the univariate analysis.
Thus, the following variables were included: gender, age, site of the primary tumor, clinicopathological type, Breslow thickness and ulceration. Therefore, 244 patients with complete information regarding all six variables above mentioned were analyzed.
Male gender (p=0.0222), nodular clinicopathological type (p=0.0196), Breslow thickness >4 mm (p=0.0011) and ulceration (p=0.0391) were considered risk factors for the occurrence of metastasis of primary cutaneous melanoma. Tumor thickness between 1 and 4mm was close to statistical significance (p=0.0628). The acral clinicopathological type did not reach statistical significance (p=0.4857) (table 3). The model’s residuals test showed p=0.611.
Cox multivariate analysis of the risk factors for primary cutaneous melanoma metastasis of 244 patients seen at the services of dermatology and surgical oncology (1995-2012)
| Variable | Hazard Ratio* | CI 95% | P value |
|---|---|---|---|
| Gender | |||
| Male | Reference** | ||
| Female | 0.46 | 0.24-0.90 | 0.0222 |
| Clinicopathological type | |||
| Superficial spreading and lentigo maligna melanoma | Reference | ||
| Nodular | 2.89 | 1.19-7.03 | 0.0196 |
| Acral | 1.50 | 0.48-4.72 | 0.4857 |
| Breslow thickness | |||
| ≤1mm | Reference | ||
| 1,01 to 4mm | 3.00 | 0.94-9.57 | 0.0628 |
| >4mm | 7.85 | 2.27-27.16 | 0.0011 |
| Ulceration | |||
| No | Reference | ||
| Yes | 2.14 | 1.04-4.40 | 0.0391 |
Melanoma is an aggressive skin cancer, characterized by the high risk of metastasis and death.1 Although it represents only 4% of skin cancers in Brazil, it is responsible for the death of almost 50% of these patients.2
Data from 780 patients with the diagnosis of melanoma confirmed by anatomopathological examination seen at 2 reference centers participating in the study between 1995 and 2021 were collected. It is a large sample of cases included in a single Brazilian study.
In the sample studied, male gender, nodular clinicopathological type, Breslow thickness >1mm and the presence of ulceration were considered prognostic factors for the occurrence of metastasis.
Other authors also found a poorer course of melanoma in men.6,7 According to Pollack et al.,4 men had a lower survival at 1 and 5 years, regardless of the age at melanoma diagnosis. Vries et al.8 showed that men have a 1.87-fold higher risk of death by melanoma than women, even after adjusting for other risk features such as age, site of the primary tumor, clinicopathological type and Breslow thickness. Recently, Liljana Mervic,9 who followed 7,338 patients in a German university, showed that women had lower risks of melanoma spread and a longer interval between the primary tumor and the first metastasis. Besides, that study showed that females develop metastasis with a more favorable prognosis, such as satellite or in-transit metastasis, when compared to those of males.
The reason for the lower risk of melanoma spread in women is still unknown. A possible explanation for this could be the fact that women pay more attention to their own bodies and seek medical care earlier.10 Hormonal influence was also postulated as responsible for a longer survival in women, but recent studies suggest that melanoma is not hormone-dependent.11,12 In a recent study, Joosse et al.reported that oxygen free radicals, such as the superoxide anion and hydrogen peroxide, are involved in the mealanogenesis of the tumor.13 The authors postulated that the oxidative stress, more pronounced in men, could be responsible for the increased ability of melanoma to spread.
Patients with nodular melanoma had a 2.89-fold higher risk for disease spread when compared to the superficial spreading and lentigo maligna melanoma types, parameters used as reference and analyzed as group, independently from the other variables analyzed. The second highest risk type was acral melanoma. However, in the multivariate analysis, it did not reach statistical significance (HR: 1.50).
In 2010, Kunte et al.14 studied the risk factors for lymph node metastasis in patients with melanoma. The nodular clinicopathological type was the second most important prognostic factor for lymph node involvement, overcome only by tumor thickness. The same study showed that the nodular component in any other clinicopathological type increased the likelihood of lymph node involvement. Superficial spreading melanomas without nodular component had an OR of 0.56, and those with a nodular component had an OR of 0.78. In this study, acral melanoma was also the second clinicopathological type of worse prognosis. Lindholm et al.7 prospective cohort also pointed towards a lower survival for patients with nodular melanoma. It is thought that the nodular type presents a worse prognosis due to its vertical growth, leading to a premature arrival of tumor cells in the lymphatic and blood vessels of the dermis.15-17 Distinct genetic characteristics can also explain the more aggressive course of this tumor. In 2008, Viros et al.demonstrated a high frequency of BRAF mutations (68.8%) and a low frequency of NRAS mutations (12.5%) in 16 patients with nodular melanoma.18 However, the influence of genetic abnormalities in the prognosis of melanoma is not yet established.
The acral type is also considered an independent risk factor for the occurrence of metastases. According to Garbe et al.,nodular and acral melanomas show a reduction in survival of 10 years, although its influence was smaller than other parameters as thickness, gender and site.19 The study that included the population of the program SEER (Surveillance, Epidemiology and End Results), with 51,704 patients of 13 different American institutions, indicated that acral melanoma was the one with lower 5-year and 10-year survival, from 1986 to 2005.9 The study by Chang et al.,16 which included 181 Chinese patients, phototype III and IV (according to Fitzpatrick’s classification), showed that acral and nodular melanoma were risk variables for the reduction of 5-year survival. Nonetheless, the authors affirmed that the importance of the clinicopathological type was inferior to the other variables such as gender, stage of the disease and tumor thickness, due to the reduction in the values of the HR from the univariate to the multivariate analysis.
Of all variables analyzed in this study, Breslow thickness was considered the most important for the occurrence of melanoma metastasis. As the thickness of the tumor increased, so did the risk of metastasis (p=0.0628 for tumors between 1-4mm and p=0.0011 for tumors >4 mm). Such result confirms the findings of various authors since the description of this variable by Breslow in 1970.20 It is believed that thicker lesions can represent more advanced tumors with more intrinsic biological aggressiveness than those with radial growth only.21,22
According to Brauer et al.,thick melanomas increase the risk of early metastasis (between six months to three years of the initial tumor), compared to late metastases, that occur after eight years of the primary tumor.23 An increased risk of local or in-transit metastasis was seen with increased melanoma thickness in the study by Stucky et al.24 According to Faries et al.,there was an increased risk of occult lymph node recurrence with thicker Breslow in patients with thin melanomas (<1mm).25 The case-control study conducted by Sartore et al.detected an increased risk of sentinel lymph node micrometastasis with the increased thickness of the primary tumor. 26
The presence of ulceration was also considered an independent risk factor for the development of melanoma metastasis in this study. Many authors confirmed the importance of ulceration in the prognosis of melanoma and the reduction in disease-free survival and survival of patients.27-29 According to Balch et al.,the presence of ulceration in patients with localized melanoma reduces survival in 5 years from 80% to 55%.30 The authors also observed that lesions with histological ulceration are associated to nodular and thicker tumors. Eigentler et al. showed the reduction in the 10-year survival in patients with T2 and T3 tumors (stage I) when ulceration was present. However, this variable did not influence T1 (≤1mm) and T4 (>4mm) tumors.31 Staging based on Breslow thickness and ulceration was the variable that most influenced 10-year survival in patients with localized melanoma in the study by Francken et al.32
According to the univariate analysis, the presence of mitoses in the histopathology was a statistically significant variable for the occurrence of metastasis in patients (p<0.0001). However, it was not possible to analyze it with the Cox method, because there was no metastasis in patients without detectable mitosis within the tumor. This invalidated the statistical method and the variable was excluded from the multivariate analysis. We can assume that, due to the low p value in the univariate analysis, the presence of mitosis is important for the prediction of metastasis in the study population.
According to Murali et al.,the presence of mitosis correlated with the reduction in the time for disease recurrence.33 The mitotic index was also significantly correlated to tumor thickness (Breslow thickness) and to the presence of ulceration, but was not independently significant in the overall survival of patients. Nagore et al.34 also detected a higher risk of metastasis in those tumors with mitosis, as well as Karjalainen et al.35 According to Gimotty et al., who studied 396 patients with thin melanoma, the patients diagnosed with metastasis within their tumors had a higher risk of recurrence in 10 years than those with a mitotic index of zero (HR=6.7).36 Svobodová et al. demonstrated that the presence of mitosis in the histopathology was associated to the reduction in disease-free survival (RR=1.911).27 It is postulated that the mitotic index is related to a worse melanoma prognosis because it reflects a superior proliferative index, what would facilitate the occurrence of more aggressive cellular clones with a higher potential to create metastases.34,37
The results of this study show that age was not an independent variable to predict the risk for metastasis (p>0.05). Even though the authors have not shown the influence of age in the recurrence of melanoma, what can actually have happened with the population in this study, another factor could explain the lack of association with age in the study sample: the lack of distinction between locoregional and distant metastases, grouped in a single outcome.38,39 Some authors postulate that the occurrence of lymph node metastasis is more common in younger individuals, and in contrast distant dissemination of the disease is lower and survival is higher in this group of patients.28,40,41 Kretschmer et al. showed that patients younger than 40 years had a higher risk of sentinel lymph node metastasis.42 However, this age group had a lower risk of local and distant disease, besides a longer specific survival. Thus, the highest rates of lymph node metastasis that occur in younger patients could have been similar to the higher rates of distant metastasis in older patients in this study, affecting the final result.
In this study, family history of melanoma did not alter the disease-free survival (p=0.985). Similarly, Hornbuckle et al. did not show differences in recurrences, in the pattern of metastasis distribution and survival among patients with and without family history of melanoma either.43 Of note, the sample of patients with metastasis and family history of melanoma of this cohort was restricted: only 11 of the 135 patients with metastasis had family members with the disease. This limits the interpretation of the results regarding this variable. Considering the studies published to date, we cannot affirm that a positive family history of melanoma is a risk factor for disease progression.
This study did not show differences in the spreading of the disease among patients with axial tumors (head, neck and trunk) compared to those with tumors on the extremities (upper and lower limbs). Other authors did not find association between the site of the primary melanoma and disease progression either.44 According to Faries et al., the occurrence of lymph node metastasis was not associated to the site of the primary tumor.25 Patients with localized melanoma on the head and neck did not show a reduced survival when compared to those with tumors on other sites, as reported by Hoersh et al. The appearance of metastases was not influenced by the site of the primary tumor in the study by Kelly et al. M-raz Gernhard et al. did not find an association between the site of melanoma and the occurrence of lymph node micrometastasis. In patients with thick melanoma, there was no difference in the reduction of disease-free survival between those with axial tumors and those with tumors on the extremities, according to Kim et al.45-48
Other considerationsIt is worth highlighting that 266 (34.1%) patients were excluded from the final statistical analysis for not fulfilling the established inclusion criteria. Around half of these (137 individuals) had non-invasive primary cutaneous melanoma, evidencing that only 17.6% of the patients in the study were diagnosed in the early stages of the disease (28.3% of the 212 patients admitted in the service of Dermatology and 13.6% of the 568 patients in the service of surgical oncology. The result contradicts other studies in the literature, that point to a diagnosis of in-situ melanoma in 25 to 30% of patients.4,49 This result can be explained by the occurrence of a selection bias due to the inclusion of a large number of patients seen at the service of surgical oncology, that are referred in more advanced stages of melanoma for the surgical treatment of locally advance or metastatic disease. Analyzing only the patients seen by dermatologists in the reference service of dermatology at the university hospital, the early stage diagnosis is similar to the results found in the literature (28.3%).
The admission of patients in the service of surgical oncology in this study could also explain the increased number of cases diagnosed with regional or distant metastases. Of the 514 patients included in the final analysis, 135 (26.3%) had metastatic disease. Literature data indicate that 10% of patients with invasive primary cutaneous melanoma progress with metastasis.1,4,49
Another observation to be considered is the reduction in the total number of patients submitted to the multivariate analysis with the Cox method. Since the study covered a period of 17 years and only recently factors such as ulceration and mitotic index were included in the AJCC staging of the disease, only 244 patients (47.5%) of the total of 514 had data on the six prognostic factors for the final statistical model, with statistically significant results with the univariate analysis. 1,50
Another limitation of this study was not performing sentinel lymph node biopsy for all patients with melanomas with thickness between 1 and 4mm, what could have caused a reduction in the number of cases diagnosed with regional metastasis. This is due to the participation of patients from the public system that still lacks resources to perform the test in all indicated cases.
The anatomopathological diagnosis of melanoma was performed by only one experienced dermatopathologist in the service of dermatology. The diagnosis of those seen at the service of surgical oncology was performed in private laboratories and reviewed by a pathologist with experience in melanocytic lesions. Even though the anatomopathological examination performed by distinct examiners can affect the description of the histopathologic features of the tumor, we believe that this has not influenced the final results in the study, because only two experienced pathologists were responsible for the diagnosis of the cases.
It is important to highlight that the present study grouped the four types of metastasis (satellite, in-transit, lymph node and distant) in a single outcome. This can lead to dissonant results with studies previously published, where the metastases are studied separately according to the affected site.
ConclusionMale gender, nodular clinicopathological type, Breslow thickness >4mm and the presence of ulceration were considered risk factors for the occurrence of metastasis in patients with primary cutaneous melanoma in this study.
Financial support: None.
Conflict of interest: None