Leprosy is a chronic infectious disease caused by Mycobacterium leprae, which affects peripheral nerves, skin and mucous membranes. The impairment of neural function as well as sensory or sensory-motor disabilities in leprosy continue to be a problem that requires careful attention in the management of patients with the aim to avoid or minimize their progression to prevent sequelae. One of the most common characteristics of these ulcers is the tendency to chronicity, with variable therapeutic response. In this article, we shall discuss the therapeutic management of thirteen trophic leprosy ulcers in eight patients using polyhexanide 0.2% products.
Hanse’s disease is a chronic infectious disease caused by Mycobacterium leprae, that affects mainly the skin and peripheral nerves and can lead to the development of incapacitating physical disabilities and potentially visible disfigurations.1
Leprosy ulcers are usually plantar, due to the neuropathic effects on the feet, which increase the risk of trauma and contribute to the chronicity in numb areas caused by the pressure of stepping.2 Many social repercussions, such as unemployment and abandonment are reported.3 They are considered one of the most common and severe complications of leprosy, and are commonly infected by other bacteria (making the healing process harder) and are usually recurrent, which will increase the incidence of physical disabilities.4,5
Different treatment methods have been used in the management of these ulcers, but the results are often unsatisfactory. The chronicity of the lesions results in high economic and social costs These wounds represent an important public health problem, since they require a prolonged and specialized outpatient treatment that must be combined to a multidisciplinary team.2,3,6
A total of 8 patients underwent treatment of a total of 13 leprosy trophic ulcers in an open, uncontrolled study for the evaluation of the therapeutic response to polyhexanide 0.2% (PHMB), once daily. In the follow-up protocol, local surgical debridement with re-exposure of dermal tissue plus biweekly clinical assessment, daily dressing change with application of the product and moisturizing of the adjacent skin were included. Radiographs of the feet were requested in order to rule out osteomyelitis and other bone abnormalities. All patients signed a consent form and the study was approved by the Ethics in Research Committee (ERC) of the department. The availability of the product was guaranteed by Walkmed® laboratories.
The classification of pressure ulcers recommended by the National Pressure Ulcer Advisory Panel (NPUAP) was used to define the evolutionary stages of the ulcers, represented in grades: I (intact skin with non-blanching erythema), II (partial-thickness loss of skin with exposure of the dermis), III (full-thickness loss of skin) and IV (full-thickness loss of skin and loss of tissue).7 In the pre-treatment phase, three ulcers were classified as Grade I (23%), five in Grade II (38.5%) and five in Grade III (38.5%).
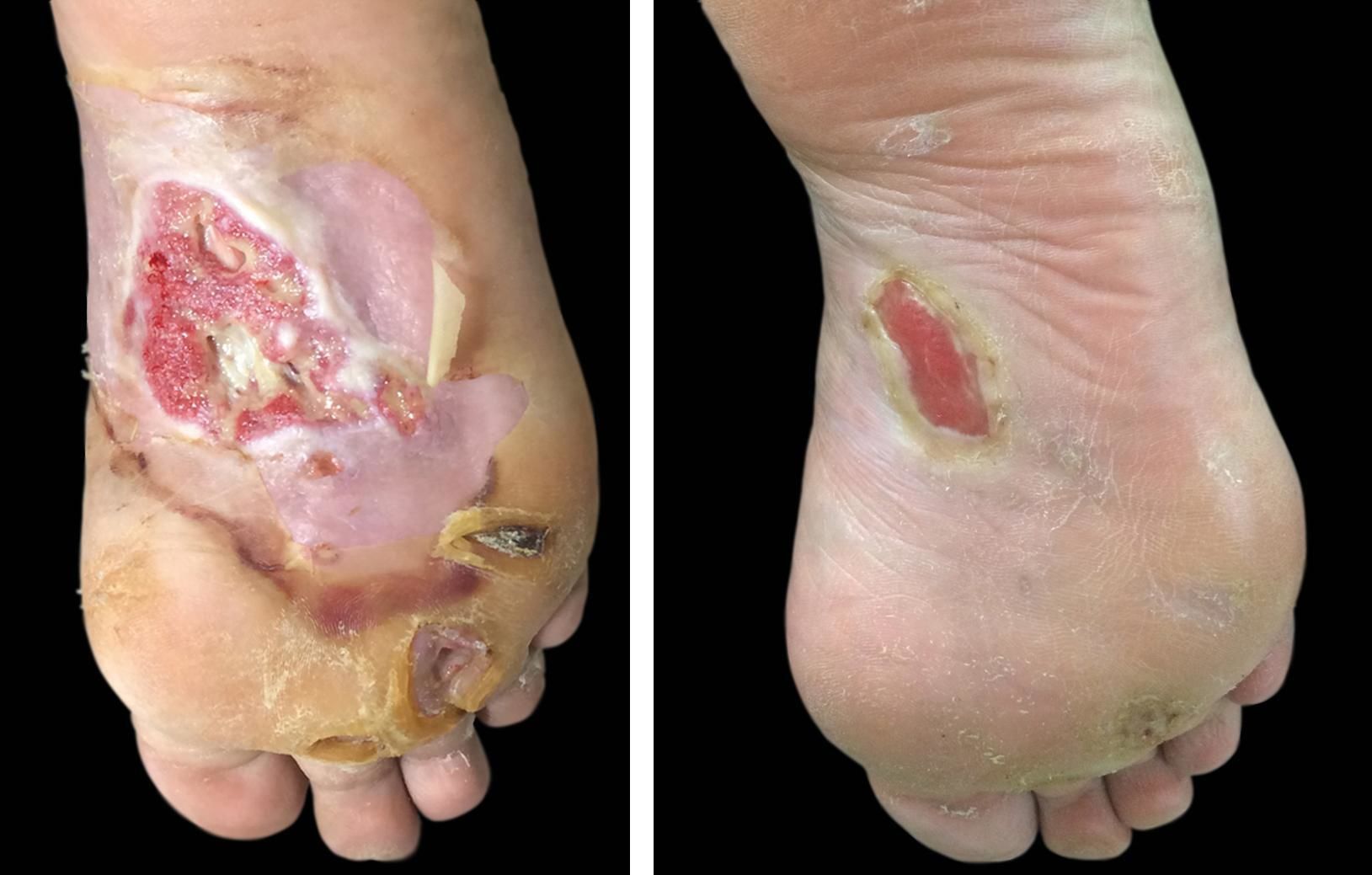
The therapeutic evolution of two patients can be seen in the initial and final assessment of the lesions (Figures 1 and 2).
Chronic ulcers are an important public health problem in Brazil and depend on the care of trained professionals. There is demand for specialized centers and the majority of patients is not able to have adequate treatment and follow-up.
Personal factors, regular consultation and treatment, self-care and guidance on the use of proper shoes are directly responsible for the end results achieved.2,3,6 The patients were referred to a service specialized in the fabrication of insoles and shoes that allowed a decreased pressure of the tissues on the bones.
The colonization of ulcers is prevalent and is described as a potential impairment to the healing process.5 The antimicrobial management of the wounds is in need of new solutions against microbes and their biofilms. Systemic antibiotics rarely penetrate the biofilms and their use is limited by the increased bacterial resistance. Topical antibiotics can easily lead to sensitization. Disinfection is considered the method of choice for the treatment of bacteria present in wounds.8
PHMB is an antimicrobial compound, appropriate for the clinical use in critically colonized or infected, acute or chronic wounds.9 It has a broader spectrum of action, cellular and tissue tolerability, low risk of contact sensitization, has no evidence of the development of microorganism resistance and also reduces the load of pathogens in critically colonized or infected wounds. It is not the only therapeutic option, however, is a promising substance.8,9
All 13 trophic ulcers were located on the feet, 8 on the plantar region (62%), 1 on the hallux (8%), 2 on the heel (15%) and 2 on the ankle (15%). They were all single, with the exception of 3 patients with 2 concomitant ulcers and 1 patient with 3. One of the patients had the lesion for 10 years and had undergone other treatment with no success. The 13 had complete regression in a period that ranged from 4 to 13 months of treatment, with no recurrence till present while on post-treatment clinical follow-up every 4 to 12 months.
Ineffective treatment of the trophic ulcer implies in complications for the patient such as osteomyelitis or even amputation in more severe cases. For the case on the screen, we demonstrate that the treatment of leprosy ulcers with PHMB is promising, achieving great results with this study group. Multidisciplinary follow-up and patient compliance were essential for the success of the treatment. In the literature, the discussion on the best therapeutic management of these wounds is discordant and larger and more controlled studies are needed.10
This preliminary study has limitations in regards to sample size, the study design and the selection method, that were subject to biases. New controlled studies are needed to clarify the efficacy of the use of PHMB on the management of trophic ulcers. However, we can conclude that the group evaluated had promising results with the proposed therapeutic option.
Financial support: None.
Conflict of interest: None.