Toxic epidermal necrolysis is a condition with massive keratinocyte apoptosis, and it is associated with high mortality rates. Fulvestrant, an estrogen receptor antagonist, is indicated in the treatment of estrogen receptor-positive metastatic breast cancer in postmenopausal women. To our knowledge, this is the first described case of toxic epidermal necrolysis due to fulvestrant. A 56-year-old woman received 500 mg of intramuscular fulvestrant monthly for metastatic ductal carcinoma of the breast. Five days after the first dose, the patient presented with a maculopapular rash that evolved to blisters, and a detachment of the epidermis in over 30% of the total body surface area. Histological analysis was compatible with toxic epidermal necrolysis. Fulvestrant was discontinued, topical management and supportive care were initiated.
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare conditions characterized by apoptosis, as well as detachment of the epidermis and mucous membranes.1 It results from type IV hypersensitivity reactions.2 In these cases, there is elevation of the Fas ligand, which binds to Fas (its receptor of keratinocytes) and induces massive keratinocyte apoptosis.1
SJS and TEN are frequently drug related. The most commonly responsible agents are sulfonamides, allopurinol, antiepileptic, and non-steroidal anti-inflammatory drugs (.Chart 1).3
SJS and TEN are currently regarded as different degrees of the same disease spectrum. These conditions differ by the extent of epidermal detachment; while TEN involves more than 30% of the total body surface area, SJS involves less than 10%.1 A high diagnostic suspicion for TEN is important, as the associated mortality rates can be as high as 25-35%. Withdrawal of the responsible drug is crucial in the prognosis.2
Fulvestrant is an estrogen receptor antagonist. It is indicated in the treatment of metastatic breast cancer in postmenopausal women when the tumor expresses estrogen receptors and there is a lack of response to other anti-estrogen therapies, such as tamoxifen. Fulvestrant exerts its antitumor activity by competing with endogenous estrogen to bind to the estrogen receptor. After binding, fulvestrant prevents receptor dimerization, effecting receptor degradation and transcription inhibition, ultimately inhibiting the growth of human breast cancer cells.4
To our knowledge, this report presents the first described case of TEN due to fulvestrant.
CASE REPORTA 56-year-old female with a history of ductal carcinoma of the breast underwent a mastectomy and ipsilateral axillary lymph node resection, and she also received a chemotherapy regimen of docetaxel, adriamycin, and cyclophosphamide. The patient underwent radiotherapy and hormone therapy with tamoxifen 6 years ago. Two years ago, she relapsed and presented with lung and bone metastases. She was then treated with capecitabine. Recently, she presented with deviation of the oral commissure and was diagnosed with brain metastasis. Given the lack of response to treatment, and since the tumor expressed estrogen receptors, she was started on 500mg of fulvestrant, which was administered intramuscularly on a monthly basis.
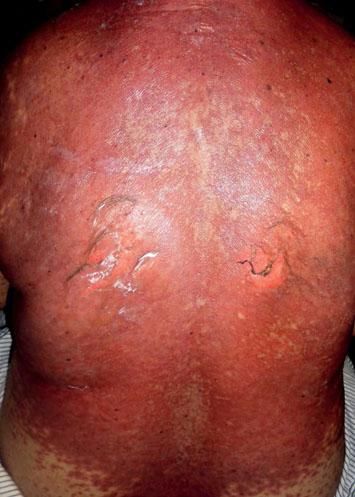
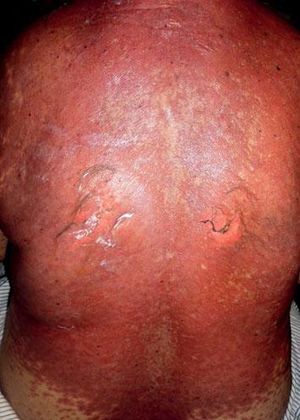
Five days after the first dose, the patient presented with a morbilliform rash on the face and trunk. Cutaneous involvement had been preceded by a fever of up to 38°C. She had not taken any other new drugs. In the next week, the lesions extended to the patient’s extremities, affecting the palms and soles. At this point, convergent, violaceous, and atypical target lesions could be seen. Throughout the second week, these lesions evolved to flaccid blisters with detachment of the epidermis. There was involvement of over 30% of the total body surface area (Figure 1). The patient presented with a positive Nikolsky’s sign and developed hemorrhagic erosions in the nasal and labial mucosae. Other organs were not affected.
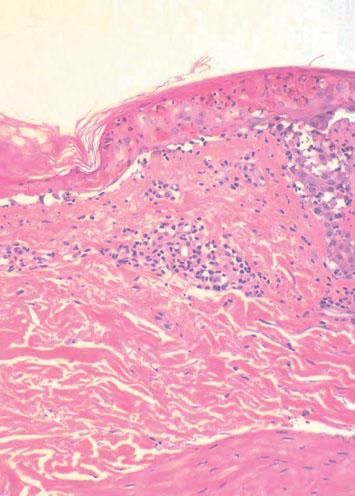
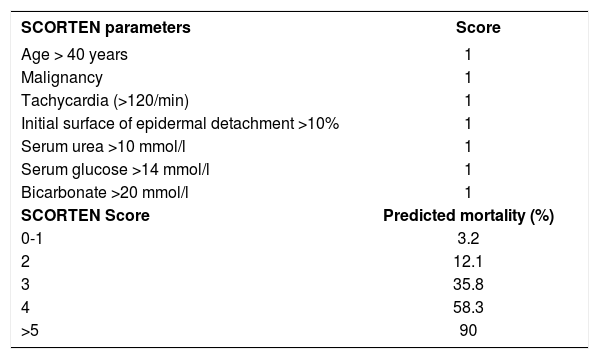
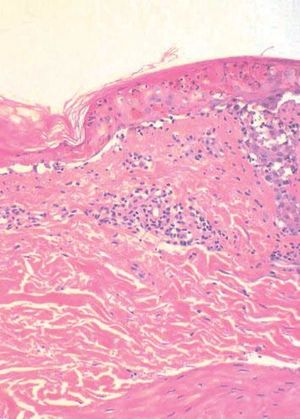
The patient was hospitalized to control the balance of fluids, electrolytes, and vital signs. On admission, the patient’s bloodwork revealed normal blood cell count, normal levels of proteins and ions, and lactate dehydrogenase levels of 1,215 U/L. A skin biopsy was taken and fulvestrant treatment was suspended. The biopsy revealed epidermal necrosis (with Civatte’s bodies) and subepidermal detachment (Figure 2). The SCORTEN scale was applied (56 years, malignancy of breast, heart rate 140 bpm, initial surface of epidermal detachment 5%, urea 60.6 mg/dl, glucose 137 mg/dl, bicarbonate 36.8 mEq/l), predicting a mortality of 58.3% for a SCORTEN of 4.
Given the presence of an underlying disease and the lack of evidence-based scientific data on the use of systemic therapy for the treatment of TEN,1,2 conservative management with topical treatment and supportive care was initiated. The patient demonstrated favorable evolution of the cutaneous lesions and was discharged within 1 month without TEN-related sequelae. Fulvestrant was replaced by vinorelbine. Unfortunately, the patient passed away after 1 year due to reasons unrelated to TEN.
DiscussionTEN is part of the spectrum of erythema multiforme major. The most common cause of this disease is drug use. SCORTEN is a tool that helps to predict the risk of death according to the parameters listed in table 1.3
SCORTEN severity-of-illness score
| SCORTEN parameters | Score |
|---|---|
| Age > 40 years | 1 |
| Malignancy | 1 |
| Tachycardia (>120/min) | 1 |
| Initial surface of epidermal detachment >10% | 1 |
| Serum urea >10 mmol/l | 1 |
| Serum glucose >14 mmol/l | 1 |
| Bicarbonate >20 mmol/l | 1 |
| SCORTEN Score | Predicted mortality (%) |
| 0-1 | 3.2 |
| 2 | 12.1 |
| 3 | 35.8 |
| 4 | 58.3 |
| >5 | 90 |
Early withdrawal of the suspected drug is essential. Patients should be managed in highly specialized centers, such as burn units. An optimal electrolyte balance should be ensured through an electrolyte solution and albumin solution. Nutritional support with a hypercaloric and hyperproteic diet is advised to prevent protein loss and promote healing.5
Skin care is based on minimizing trauma, puncturing blisters, and using antiseptics and non-adherent mesh gauze for erosions. Bland ointments can be used for lip erosions and mouthwash disinfectant for oral erosions. Ophthalmologic care is necessary, if there is eye involvement. Sitz baths are beneficial for genital erosions.5
Regarding systemic treatment, corticosteroids and intravenous immunoglobulins (IVIG) are the most commonly used drugs.1 The use of IVIG is extended by the discovery that IVIG blocks the Fas-mediated necrosis of keratinocytes in vitro.6 However, the use of IVIG in clinical practice is controversial. Recently, a meta-analysis that evaluated 17 studies concluded that IVIG does not decrease mortality.7
The use of steroids in the treatment of TEN is based on case reports and case series. An increased rate of infections, a delay of re-epithelialization, and prolonged hospitalization has been reported with the use of steroids.8 Steroids have not been shown to have any effect on mortality rates.9
Valeyrie-Allanore performed a clinical trial to evaluate the possible benefits and safety profile of cyclosporine in patients with SJS/TEN. They concluded that cyclosporine decreased expected death rate according to SCORTEN.10
Given the presence of the underlying disease and the lack of a firm evidence-based recommendation for the use of systemic therapy for the treatment of TEN, the patient was exclusively managed with topical treatment and supportive care with a favorable outcome. It should be noted that the skin reactions induced by fulvestrant are rare.4
We report the first case of fulvestrant-induced toxic epidermal necrolysis. Withdrawal of the suspected drug, intensive supportive care and fluid management in specialized centers are essential in the management of TEN.