Fogo selvagem or endemic pemphigus foliaceus is an autoimmune acantholytic anti-cadherin bullous disease that primarily affects seborrheic areas, which might disseminate. Brazil has the world’s largest number of patients, mainly in the Central-West region, but the disease has also been reported in other South American countries. It affects young people and adults who have been exposed to rural areas, with occurrence of familial cases. Anti-desmoglein-1 autoantibodies are directed against desmosomal structures, with loss of adhesion of the upper layers of the epidermis, causing superficial blisters. The etiology is multifactorial and includes genetic, immune, and environmental factors, highlighting hematophagous insect bites; drug-related factors are occasionally involved. Flaccid blisters readily rupture to yield erosive-crusty lesions that sometimes resemble seborrheic dermatitis, actinic keratosis, and chronic cutaneous lupus erythematosus. The clinical presentation varies from localized to disseminated lesions. Clinical suspicion should be confirmed with histopathological and immunofluorescence tests, among others. The progression is usually chronic, and therapy varies according to clinical presentation, but generally requires systemic corticosteroid therapy associated with adjuvant immunosuppressive treatment to decrease the adverse effects of corticosteroids. Once the disease is under control, many patients remain stable on low-dose medication, and a significant proportion achieve remission.
The term pemphigusrefers to a group of cutaneous diseases clinically characterized by the manifestation of vesicle-blisters and/or erosions of the skin and/or mucous membranes. The disease is characterized histologically by the presence of acantholysis, that is, the separation of the keratinocytes resulting in the formation of intraepidermal cleavage, and/or immunologically by the detection of autoantibodies against intercellular adhesion molecules called desmogleins (Dsg), which are transmembrane desmosomal glycoproteins.1,2
Among the pemphigus presentations, we highlight pemphigus foliaceus (PF), with exclusive cutaneous involvement due to the production of antibodies against desmoglein 1 (Dsg1), and pemphigus vulgaris (PV), which manifests in two forms: the mucous type, due to the synthesis of antibodies against desmoglein 3, and the mucocutaneous type, with concomitant production of anti-Dsg1.2,3Table 1provides a summary of the clinical and laboratory findings of the pemphigus group and its variants.4
Summary of the clinical and laboratory findings of the pemphigus group and variants
| PEMPHIGUS | Variant | Antibody/ Subclass | Antigen / Molecular Weight | Level of the acantholisis | Mucosal lesions | Localization of the lesions |
|---|---|---|---|---|---|---|
| Foliaceus (PF) | Classic or Cazenave Endemic or Fogo Selvagem Erythematous (Senear Usher) | IgG / IgG4 IgG / IgG4 IgG / IgG4 ANA (+) | Dsg 1 / 160 kDa Dsg 1 / 160 kDa Dsg 1 / 160 kDa | SubcornealSubcorneal Subcorneal | NoNoNo | Seborrheic areas, can disseminate Seborrheic areas, can disseminate Malar regions +symptoms of SLE |
| Vulgaris (PV) | Mucosal dominant Mucocutaneous Vegetans | IgG / IgG4 IgG / IgG4 IgG | Dsg 3 / 130 kDa Dsg 3 / 130 kDa Dsg 1/160 kDa | Suprabasilar Suprabasilar | YesYesYes | Oral mucosa is more common Mucocutaneuous lesions Mucosal and intertriginous areas |
| Drug Induced | Type PF Type PV | IgG | Dsg 1/160 kDa Dsg 3/130 kDa | PF-like PV-like | No Yes | PF like PV like |
| Paraneoplastic | Paraneoplastic | IgG | Dsg 3/130 kDa Dsg 1/160 kDa *Plakin family | Suprabasal - erythema multiforme /lichen planus-like | Yes | Severe mucositis |
| By IgA | Subcorneal dermatosis Intraepidermal neutrophilic | IgA IgA | Desmocollin 1 / 110/100 kDa ? | Subcorneal pustules Suprabasilar pustules | Rare Rare | Vesicopustules on axilla and groin Vesicopustules on axilla and groin |
IgG: immunoglobulin G; Dsg: desmoglein; ANA: antinuclear antibody; SLE: systemic lupus erythematosus.
The endemic variant of PF is known as fogo selvagem (FS), which means “wildfire” in the Portuguese language. FS shares clinical, histopathological, and immunological characteristics with classical PF and is distinguished from it by epidemiological aspects, namely higher frequency in children and young adults in rural areas and in regions geographically known by high incidence of FS, besides the presence of familial cases. The symptoms resemble those of burn injuries. Most FS patients live in rural areas, which explains how the disease came to be known as “fogo selvagem” or “wildfire”, a term that has been established in the medical literature.4-6
Until the 1940s, the diagnosis was eminently clinical. Since then, however, with the aid of histopathology, FS came to be described as an acantholytic bullous disease.7In 1964, Beutner and Jordon detected antibodies in the intercellular space of the epidermis, and pemphigus came to be defined as an autoimmune acantholytic bullous disease.8 Desmoglein 1 (Dsg1) is the autoantigen recognized by the autoantibodies of patients with FS or PF and, as a member of the desmosomal cadherin family of cell adhesion molecules, FS is currently defined as an autoimmune anti-cadherin acantholytic bullous disease.9,10
Historical AspectsBullous eruptions were described by the Greeks, who used terms such as pemphix, pomphos, and pompholyx.11Hippocrates (460-370 BC) used the term “ pemphigodes pyretoi” to describe a type of fever in a disease not accompanied by blisters.12 The term “ febris pemphygodes” was applied in the 17th century to various bullous diseases, but De Sauvages called pemphigusa vesiculobullous disease.13 In 1777, McBride was the first author to describe pemphigus vulgaris, with the term morbus vesicularis. 14 Finally, pemphigus foliaceus was first described in 1844 by Pierre Louis Alphée Cazenave.15
Pemphigus brasiliensis was mentioned for the first time by François Boissier de Sauvages (1768) in his work Nosologia Methodica, referring to the observation made by Father Bougeant, who worked as a missionary in Brazil in 1730. In 1948, however, after a critical review, Silva concluded that it was herpes zoster.16 The first FS patient diagnosed in Brazil was reported by Caramuru Paes-Leme in 1903, causing confusion with the bullous variant of tinea corporis, that is, tinea imbricataor Tokelau. Gualberto, in 1912, corrected this diagnosis when he discovered that the description was similar to the PF of Cazenave.17
The modern history of pemphigus began with Heinrich Auspitz (1881), who described the histological findings of a bullous lesion called acantholysis.15 The diagnostic maneuver applied to pemphigus (detachment of the skin by pressing it in the vicinity of a lesion) was suggested by Nikolsky in 1896.18 The first description of FS histology was performed by a Brazilian dermatologist, João Paulo Vieira, who published the study entitled “Contribution to the Study of Pemphigus in the State of São Paulo” in 1937.19 Civatte, in 1943, differentiated PV from PF based on histology, and in 1953 Lever separated bullous pemphigoid from the pemphigus group based on clinical and histopathological findings.20 In 1964, Beutner and Jordon described pemphigus antibodies in patients with PV.8 In 1968, antibodies were detected in the intercellular space in patients with FS,21 and in 1982, the pathogenic nature of these autoantibodies was demonstrated in an animal model (BALB/c mice) by Anhalt et al.22
Some studies have analyzed the historical migration of FS in South America. The first cases were diagnosed in the State of Bahia, Brazil, then in the States of São Paulo and Minas Gerais, where two important outbreaks were observed in 1912, one in Ouro Preto and the other in Belo Horizonte, later appearing also in Mato Grosso and Goiás.23 In 1937, a lieutenant-general and army physician stationed in Ponta-Porã/MT (now Mato Grosso do Sul – MS), impressed by the number of FS patients in the region, recommended installing a hospital facility to treat FS.24 Campos (1942) cited the creation of two hospitals for FS treatment, Adhemar de Barros Hospital in São Paulo and another hospital in Ponta Porã/MS (the latter, when inaugurated, was turned over to the local community to serve as a general hospital).25
In 1949, the Pemphigus Adventist Hospital was built in Campo Grande/MS, where the topical tar product Jamarsan was used with relative success, since 65% of the FS patients had this potentially fatal disease controlled, as reported by Counter (1959).26 FS cases were reported in Argentina in 1948 and later in Paraguay. The disease was diagnosed in Peru in 1949 and in Venezuela the following year. The first cases in Colombia were reported in 1984.23 In 1995, in Tunisia, a significant incidence of PF was diagnosed in women 25 to 34 years of age without familial cases.2 Brazil has the largest historical case load, and FS has been referred to as “Brazilian pemphigus foliaceus”.21
EtiopathogenesisEpidemiological and environmental factorsPemphigus is rare worldwide, with a global incidence around 0.76 to 5 new cases per million inhabitants per year. PV is more common than PF in most countries, with the exception of Finland, Tunisia, and Brazil. In rural areas of Brazil, the ratio of FS to PV can reach 17:1. Currently, the countries with the highest reported numbers of FS cases are Tunisia and South American countries, particularly Colombia, Paraguay, Peru, Venezuela, and especially Brazil, where more than 15,000 patients were estimated as of 1989. Unlike classical pemphigus foliaceus (or Cazenave), in which the majority of patients are middle-aged and elderly, FS affects both sexes of any ethnic group, ranging mainly from preadolescents to young adults who have been exposed to rural areas, and with occurrence of familial cases. As these areas are settled, the incidence of FS gradually decreases. Furthermore, the ecological systems of FS share similarities with those of Chagasdisease and leishmaniasis vectors.1,3,5,27
Aldeia Limão Verde is a Terena indigenous reservation located in the State of Mato Grosso do Sul (Brazil), with a population of 1,300 and high prevalence (3.4%) of FS.28 A case-control study conducted in this village by the Cooperative Group on Fogo Selvagem Research warned that inhabitants of this endemic area were at risk of developing FS if their houses were built with adobe walls and thatched roofs, and that the odds of developing FS increased if they were exposed to hematophagous insects like black flies, triatomines (kissing bugs), sandflies, or bedbugs. The predominant black fly in this village was Simulium nigrimanum, a species rarely found in nonendemic areas of Brazil.27,29,30
Other studies by the Cooperative Group discovered that individuals who present the HLA-DRB1 human leukocyte antigen and who are repeatedly bitten by hematophagous insects show an increased relative risk of producing IgM and IgE autoantibodies to salivary antigens of these insects. The production of autoantibodies to the protein antigen LJM11 of the salivary glands of the phlebotomine or sandfly ( Lutzomyia longipalpis) may lead to the recognition of Dsg1 by conformational molecular mimicry through the phenomenon of intramolecular epitope spreading. Anti-Dsg1 monoclonal autoantibodies from FS patients reacted in a cross-linked manner with LJM11, and mice immunized with LJM11 produced anti-Dsg1 antibodies.31
Genetic factorsUp to 20% of genetically related family members can develop FS (up to 60% in the Limão Verde village). Several studies have shown that certain major histocompatibility complex (MHC) type II genes are often related to FS. The expression of the DRB1*0404, DRB1*1402 and DRB1*1406 alleles shows significant association with FS (relative risk = 14). The hypervariable region of the DRB1 gene of these alleles at the 67-74 region shares the same sequence of amino acids, LLEQRRAA. This shared epitope may confer susceptibility to the development of FS, as with the hypothesis for rheumatoid arthritis.32
The MHC class II trans-activator regulates the expression of HLA class II genes. HLA-DRB1 genotypes are known to have a strong influence on the risk of multifactor autoimmune diseases. The odds ratio for individuals having two susceptible HLA-DRB1 alleles is 14.1 in the presence of CIITA G/G or G/A genotypes and lower (2.2) in the presence of CIIT A/A protective genotype. Not only quantitative but also qualitative variations of HLA class II molecules have a role in the risk of developing FS.33
DrugsCertain drugs can precipitate pemphigus foliaceus, with D-penicillamine and captopril as the most frequent. In one publication, at least 7% of 104 patients receiving D-penicillamine developed PF.34Other drugs associated with PF are penicillin, cephalosporins, rifampicin, other angiotensin-converting enzyme (ACE) inhibitors (e.g., enalapril), non-steroidal anti-inflammatory drugs, tiopronin, and pyritinol, among others.35
Immune FactorsFS antigen - desmoglein 1 (Dsg1)Using immunoelectron microscopy, PV and PF antigens were identified in the desmosomes, which are the most important cell adhesion junctions of the squamous epithelium. Immunochemical characterization of pemphigus antigens by immunoprecipitation or immunoblotting with extracts of epidermis or keratinocytes in culture showed that PV and PF antigens are transmembrane glycoproteins with molecular weights of 130 kDa and 160 kDa, respectively (Table 1). Comparative immunochemical studies using anti-Dsg1 monoclonal and polyclonal antibodies found that the 160 kDa protein recognized in the serum of FS patients was Dsg1. Molecular cloning of cDNA with PF and PV antigens indicated that both molecules (Dsg1 and Dsg3) are members of the cadherin supergene family.9,36,37
Cadherins belong to the family of calcium-dependent cell adhesion molecules that play an important role in the formation and maintenance of complex tissue integrity. Based on sequential similarity, cadherins have two main subgroups, the classical cadherins (e.g., -E-, - P, -N-cadherins) and the desmosomal cadherins (desmogleins and desmocollins). All members of the cadherin family contain repeat sequences of amino acids with calcium binders in the conformation of the extracellular domains. The Dsg1 molecules are preferentially distributed in the upper layers of the epidermis, and each Dsg1 molecule is subdivided into five extracellular domains (EC1 to EC5), where the autoantibodies bind. The disease develops when autoantibodies target the amino-terminal portions of desmoglein where the important epitopes for pathogenicity (the EC1/EC2 domains) are located (Figure 1).3,9,27,38 Evangelista et al. (2018) demonstrated that FS IgG4 autoantibodies recognize a 16-amino acid peptide located on the EC1 domain of Dsg1 (residues A129 LNSMGQDLERPLELR144) (Figure 1).39,40
Molecular homology between desmoglein 1*, desmoglein 3 and E-cadherin. Desmogleins 1 and 3 are desmosomal transmembrane proteins sharing the same cadherin-like domain structure of E-cadherin. The EC1-EC2 domains of Dsg1 are recognized by pathogenic autoantibodies of FS patients, whereas Dsg3 is bound by autoantibodies from pemphigus vulgaris patients. Autoantibodies against E-cadherin have also been described in mucocutaneous PV, PF, and FS.40
Legend: Dsg 1 – desmoglein 1; Dsg 3 – desmoglein 3; E-cad – E-cadherin
Adapted from Culton, et al, 2008.40
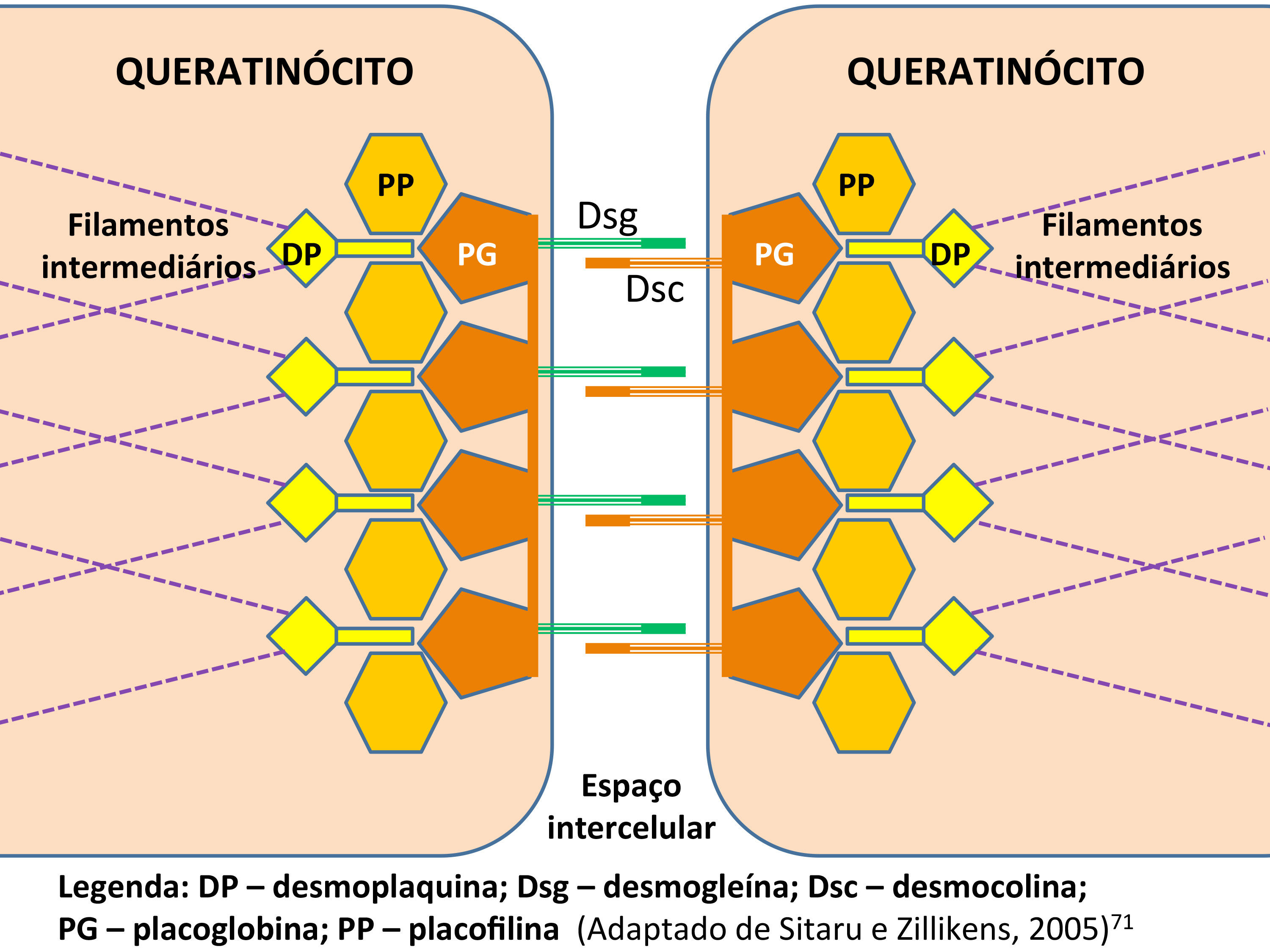
The extracellular portion of Dsg1 establishes homophilic and heterophilic interactions with the other cadherins - desmogleins 2 to 4 and desmocollins 1 to 3 - while their cytoplasmic domains bind to plakophilins 1 to 3 and plakoglobins, which connect to the desmoplakins, which in turn bind to the intermediate filaments of the cytoskeleton. This complex of proteins responsible for adhesion between the epidermal keratinocytes is called the desmosome (Figure 2).41,42
FS pathogenic autoantibodiesThe main characteristic of pemphigus is the detection of IgG autoantibodies in the intercellular spaces of the epidermis, demonstrated by immunofluorescence.7,20 Pemphigus antibodies found in the serum of patients play a primary pathogenic role in the induction of loss of cell adhesion between keratinocytes and subsequent formation of vesicle-blisters. 3,27,40
The binding between IgG and Dsg1 leads to disruption of the desmosomes located in the upper portion of the epidermis and loss of adhesion between the keratinocytes, a process known as acantholysis. 43 The location of acantholysis in pemphigus can be explained by compensation theory, according to which the level of cleavage varies according to the autoantibodies and their distribution in the epidermis.44
Dsg1 is preferentially located in the upper layers of the skin, and upon binding with anti-Dsg1 IgG, cleavage forms in the subcorneal or high granular layer. There is no mucosal involvement in FS and PF, because there is preferential expression of Dsg3 in the epithelium, which compensates for the loss of Dsg1 and allows maintenance of its integrity.44
Recognition of Dsg3 by PV autoantibodies promotes loss of adhesion between suprabasal keratinocytes, given their predominant distribution in the lower portion of the epidermis. In the mucosa, the maintenance of intercellular adhesion is more dependent on Dsg3, which is present throughout the epithelium, with less participation of Dsg1. For this reason, individuals with PV develop mucosal lesions, as well as skin involvement in cases with concomitant synthesis of anti-Dsg1 IgG, (mucocutaneous pemphigus vulgaris).44
The immune response is mediated by anti-epithelial IgG autoantibodies, predominantly of the IgG4 subclass, although IgG1 antibodies are also present. In experimental models for FS conducted in mice, in addition to IgG4, F(ab’)2 and IgG Fab fragments were also found to be pathogenic. 1,40,45
Normal individuals from endemic areas show low levels of anti-Dsg1 IgG1 and IgG4 autoantibodies, while FS patients have the same IgG1 levels, but the IgG4 response is 19-fold higher. Furthermore, in the preclinical phase and remission of FS, the anti-Dsg1 response is predominantly IgG1, contrasting with the active disease phase, in which anti-Dsg1 IgG4 antibodies are the main antibody isotype involved.46
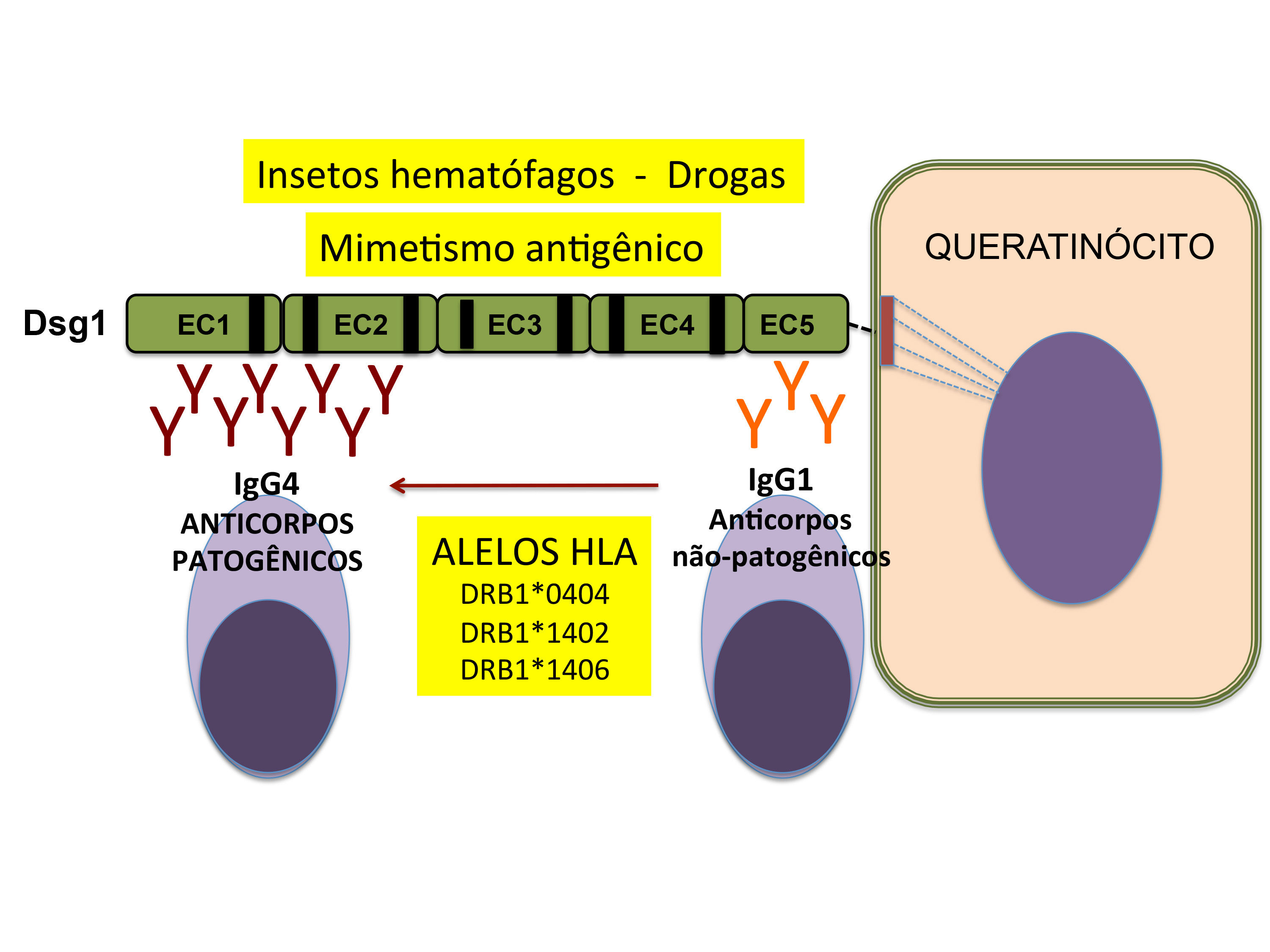
Healthy individuals living in endemic areas for FS can produce anti-Dsg1 IgE, IgM, and IgG1 autoantibodies that recognize the non-pathogenic EC5 domain. However, genetically predisposed individuals are capable of producing anti-Dsg1 IgG4 that interacts with the pathogenic domains EC1/EC2 by intramolecular expansion of the epitopes (epitope spreading), developing FS (Figure 3).47,48
Acantholysis is mediated by both extracellular (signaling-independent) and intracellular (signaling-dependent) mechanisms. The interaction between IgG and Dsg blocks the homophilic binding (Dsg-Dsg) of the intercellular adhesion molecules, in a phenomenon known as steric hindrance.49,50 In PV, this mechanism contributes to acantholysis. However, Waschke et al. demonstrated in vitro that homophilic interaction between Dsg1 molecules can be preserved even after binding with anti-Dsg1 IgG. This finding reinforces the concept that activation of intracellular pathways is also necessary to induce acantholysis in PF (and FS).41
Intracellular signaling-dependent acantholysis begins with interaction of IgG4 with Dsg1, which promotes phosphorylation of the p38 mitogen-activated protein kinase (p38 MAPK). Activation of p38 MAPK triggers the retraction of the keratin and actin filaments responsible for stabilization of the desmosomes. Disorganization of the desmosomes leads to grouping of Dsg1 molecules, contributes even further to acantholysis in PF.40,49 This mechanism is also influenced by the repertoire of polyclonal autoantibodies produced by each individual with PF. Experimental studies have shown that the combination of anti-Dsg1 monoclonal antibodies induces greater retraction of the desmosomes, when compared to the presence of a single anti-Dsg1 monoclonal antibody.50
IgG4 and IgE antibodies develop in individuals chronically exposed to environmental allergens or during immunotherapy in patients with allergic diseases. Anti-Dsg1 IgE index values of FS patients were significantly higher when compared to PF patients (from the USA and Japan) and healthy controls. Anti-Dsg1 IgE index values in healthy controls from Brazil and USA/Japan were also statistically significant. There was a correlation between IgE and IgG4 in the FS group. The triple anti-Dsg1 IgM, IgG4, and IgE response was more frequent in FS patients than in normal controls from the same endemic and non-endemic regions.46,51,52
A significantly higher frequency of anti-Dsg3 antibodies has been found (p<0.001) in healthy controls from endemic areas for FS (36%) compared to surrounding areas (6%), which supports the hypothesis that the FS antibody responds to an environmental factor in the endemic area, and that the population at risk of developing FS may also be at risk of developing the endemic form of PV, as already published.48,53
Interleukins in Fogo SelvagemSeveral studies have pointed to the role of different pro and anti-inflammatory chemokines and cytokines in the immune response of pemphigus, whose results are still controversial, while their role in the pathophysiology is unknown. Studies in PF patients undergoing treatment increased serum IL-6 and TNF-α levels when compared to healthy controls. In endemic and non-endemic PF cases, higher levels of IL-6 and TNF-α were found in patient biopsies. Timóteo et al.(2017) detected higher levels of IL-22 and CXCL8 and lower levels of IFN-γ, IL-2, IL-15, and CCL-11 in untreated FS patients, and maintained reduction of IL-22 and CXCL-10 and increased levels of IFN- γ, IL-2, CCL-5 and CCl-11 in patients undergoing treatment, suggesting that IL-22 may also play a role in the pathogenesis of FS.54-58
Clinical FeaturesThe primary cutaneous lesion is a superficial vesicle/blister, which may be filled by light or yellowish liquid, resembling an impetigo lesion. Since the lesions are flaccid, they burst easily, leaving erosive and/or erosive-crusted areas. The lesions are located in seborrheic areas, namely on the scalp, face, and upper central region of the trunk, and tend to spread distally. In all active forms of FS, the Nikolskysign (perilesional skin detachment) is easily triggered. Some authors propose two Nikolskysigns: direct Nikolskysign (type I), performed on apparently normal skin, close to lesions, which monitors disease activity; and the marginal Nikolskysign (type II or Asboe-Hansensign), performed in a perilesional area where a vesicle/blister expands on compression, serving as an adjunct to clinical diagnosis.5,30,59
No mucosal blisters or erosions are observed, even in patients with disseminated disease. The disease begins gradually in most patients, with cutaneous lesions appearing over a period of weeks to months. Rarely, FS can be acute or fulminating, with extensive and widespread blisters eroding over a period of 1 to 3 weeks. Pregnant women with FS bear normal children, except when they have the disseminated form of the disease with high autoantibody titers.5,30
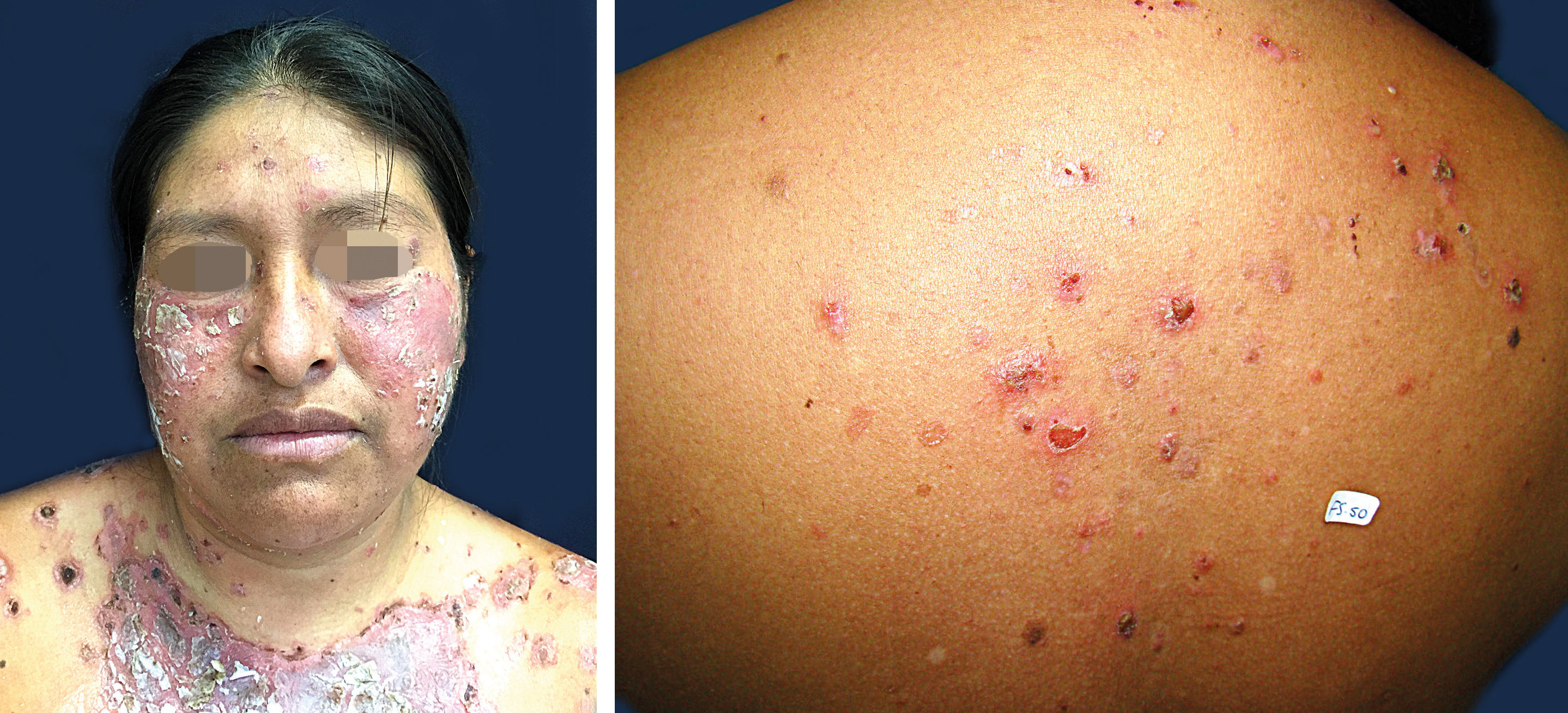
Localized forms of FSThe lesions may be small vesicles that burst easily, leaving erythematous-crusty erosions distributed on the seborrheic areas of the face and/or scalp and/or upper trunk. On the face they may be shaped like a butterfly wing (Figure 4). Sometimes, they can appear as crusty or keratotic plaques with a brownish surface. These localized FS lesions may remain unchanged for months or years, and spontaneous resolution may occur in an indeterminate number of patients. In some patients, new lesions arise centrifugally, involving the trunk and limbs. These latter presentations are called generalized FS.5,30,60
Pemphigus erythematosus or Senear-Usher SyndromeCases with scaly, crusty erythematous lesions in the malar region mimicking discoid lupus erythematosus are sometimes classified as Senear-Ushersyndrome (while some authors suggest not classifying this a separate variant). This syndrome should be reserved for patients who have laboratory abnormalities of both FS and lupus erythematosus, including histopathology and direct and/or indirect immunofluorescence (ANA positive).4,5
Disseminated forms of FSThe disseminated forms of FS can be subdivided into four distinct clinical variants (Figure 5):
I - Vesiculobullous or bullous-exfoliative: rapid onset of flaccid vesiculobullous lesions, with multiple erosions, sometimes with pustular content. When the vesiculobullous lesions develop in an annular or circinate arrangement, eroding with crusts, they resemble tinea imbricata.
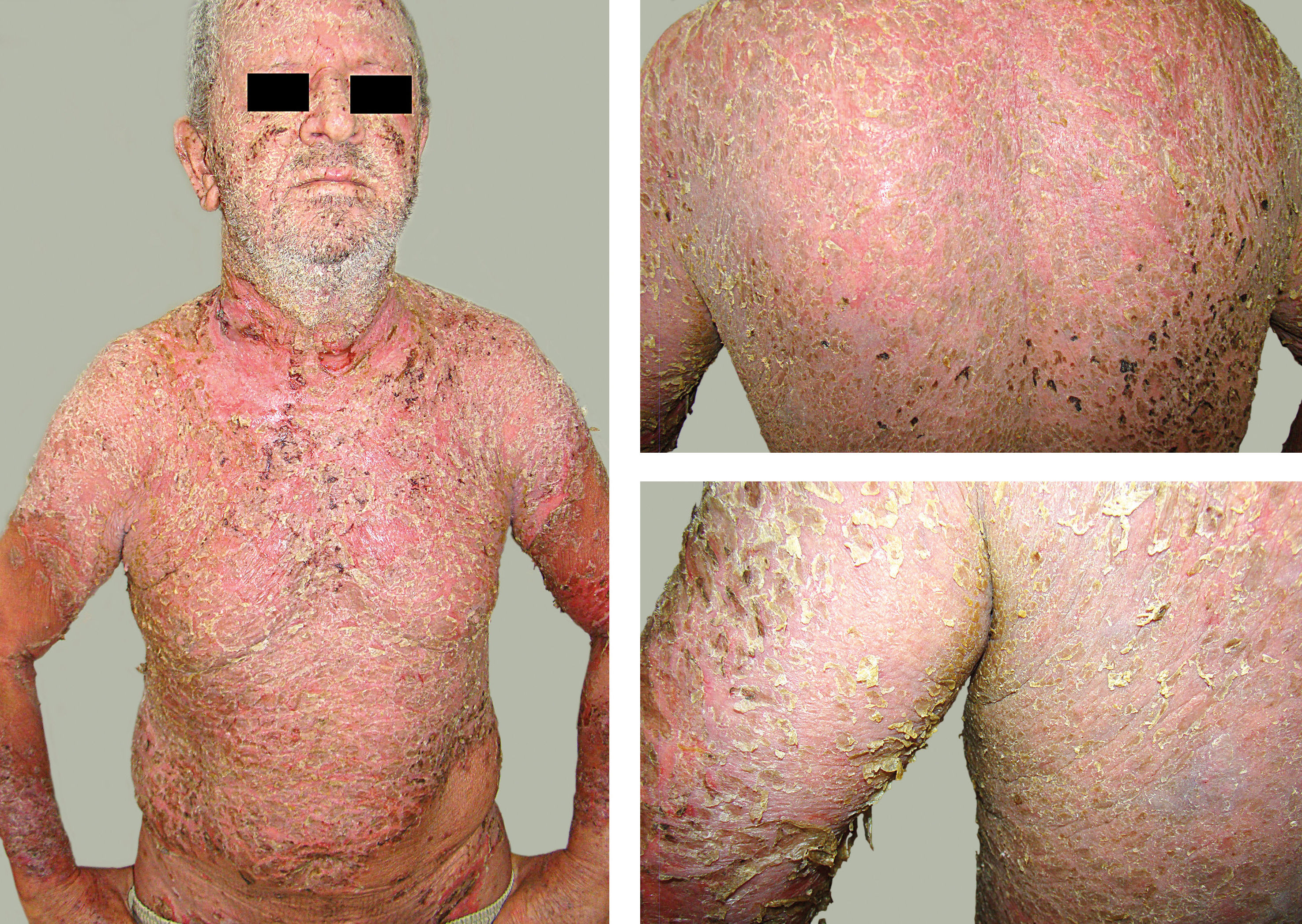
II - Erythrodermic: the entire integument is erythematous and desquamative, with the presence of erosive-exudative-crusty areas. After ruling out other causes of erythroderma, the diagnosis of FS is confirmed by laboratory tests (histopathology and immunological tests).
III - Keratotic: the treatment-resistant form, presenting elevated generalized keratotic plaques. This is a rare variant.
IV - Herpetiformis: besides FS, this variant can also manifest in PF and PV. Characterized by erythematous and urticariform plaques, with vesicles and/or pustules in a zosteriform arrangement, with pruritus present. It can precede or succeed the FS. Histopathology shows eosinophilic spongiosis and subcorneal pustules, practically without any acantholysis. Immunological results are similar to those of PF.5,30,60
Neonatal Fogo SelvagemNeonatal pemphigus foliaceus is a rare transient condition, in which neonates present clinical, histological, and immunological alterations (on DIF and IIF) consistent with pemphigus. The cutaneous alterations are erythematous-erosive-desquamative and result from transplacental autoantibodies from the mother with pemphigus. Neonatal pemphigus vulgaris is more frequent than neonatal pemphigus foliaceus, since the presence of desmoglein 3 predominates in the skin of the newborn rather than Dsg1.61 The lead author of this paper diagnosed neonatal fogo selvagem in the infant of a mother with disseminated FS and high anti-Dsg1 autoantibody titers (in press).
Differential DiagnosisLocalized forms of FS may resemble seborrhoeic dematitis, impetigo or chronic cutaneous lupus erythematosus. Disseminated forms of FS should be distinguished from impetigo, subcorneal pustular dermatosis, subacute lupus erythematosus, bullous lupus erythematosus, IgA pemphigus, and other autoimmune bullous diseases, particularly pemphigus vulgaris. In the evaluation of erythroderma of unknown cause, it is recommended to perform immunological studies to rule out the diagnosis of pemphigus. A complete review of medications should be done to exclude the possibility of drug-induced pemphigus foliaceus.
Laboratory DiagnosisThe definitive diagnosis of autoimmune bullous dermatosis requires histopathology and immunological tests (direct and/or indirect immunofluorescence and/or ELISA), besides clinical workup.3,30,62
Cytology or Tzanck test: a smear of material collected from the roof or base of the vesicle-blister is stained with hematoxylin-eosin or Leishman to visualize acantholytic cells. Acantholytic cells are keratinocytes that have lost intercellular adhesion, becoming rounded, sometimes multinucleated, and with a higher nucleus-cytoplasm ratio, giving them a dysplastic appearance. The presence of acantholytic cells in the vesicle-blister means that the bullous dermatosis is pemphigus (pemphigus foliaceus, pemphigus vulgaris, or fogo selvagem).
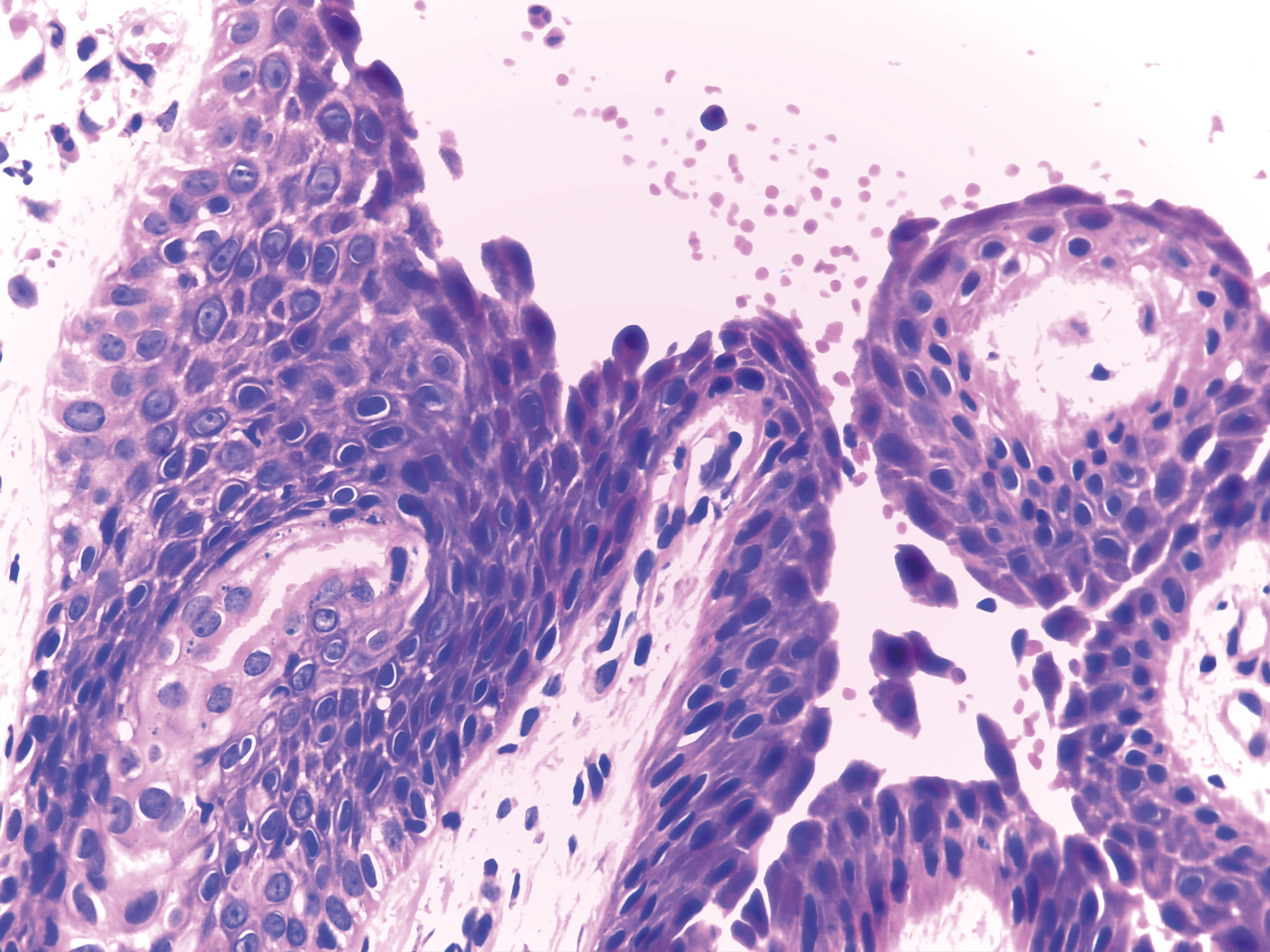
Histopathology: Subcorneal cleavage occurs (within or below the granular layer) with presence of acantholytic keratinocytes, and mixed inflammatory infiltration in the superficial dermis with eosinophils and neutrophils (eosinophils predominate in drug-induced pemphigus). Since the vesicle-blisters are superficial and flaccid, it is difficult to obtain an intact vesicle; it is recommended to collect a fresh edge biopsy (that is, part erosion and part perilesional skin) with a 4mm punch. Thus, one observes the high level of intraepidermal detachment (in FS) as well as some acantholytic keratinocytes near the roof or cleavage floor. In PV, acantholytic cleavage is suprabasal (Figure 6).
Direct immunofluorescence (DIF): This test is the gold standard, showing deposits of IgG and/or IgG4, and C3 in the intercellular spaces of the epidermis in 100% of cases with active disease. The biopsy must be taken from apparently normal skin, close to the FS lesion. If DIF is negative, the diagnosis should be questioned, except in drug-induced pemphigus, in which DIF may be negative. 63
Indirect immunofluorescence (IIF): This test is performed with the patient’s serum to detect circulating anti-Dsg autoantibodies. Its titration can correlate with the activity and extent of cutaneous involvement. In FS and PF, through immunofluorescence techniques, both direct and indirect, the fluorescence may be more evident in the upper layers of the epidermis (as well as more intense in the lower layers in PV), but in the majority of the tests with this technique, it is difficult or even impossible to differentiate FS/PF from PV (Figure 7).
ELISA (enzyme-linked immunosorbent assay): the patient’s serum is examined using recombinant desmoglein-1 as the antigen source, which has a high level of sensitivity (greater than 90%) and specificity, superior to IIF. It can also be used for the patient’s follow-up
Additional serological tests may be indicated, such as immunoprecipitation and immunoblotting, but they are limited to academic studies because of their complexity.
In summary, the laboratory evaluation to confirm the clinical diagnosis of pemphigus or any other autoimmune bullous disease includes: biopsy of injured skin (and/or mucosa if pemphigus vulgaris) for routine HE staining at the edge of the blister or erosion, with a 4mm punch; perilesional skin biopsy for DIF; and/or serum sample for ELISA and/or IIF.
ComplicationsIn extensive forms, alteration of the skin barrier may result in protein and fluid loss, electrolyte imbalance, accelerated catabolism, and increased risk of local and systemic infections. The immunosuppressive treatment used in patients with severe onset FS may predispose to opportunistic infections, sometimes fatal.
TreatmentThe treatment goal is to induce complete remission of the disease, minimizing the drug-related adverse effects.3064-67 Risk factors and comorbidities must be identified, in addition to evaluating prognosis according to the patient’s age and general condition. Regarding indications for hospital admission, the patient’s clinical condition should be assessed using the Karnovsky index, in which a value of 100% refers to a normal person, with no evidence of disease; hospitalization is indicated when the index is 50% or less (50% refers to a patient requiring considerable assistance, often medical/specialized care, and 40% refers to an incapacitated patient requiring specialized care and assistance).68
A complete review of patient’s recent medication with the potential to induce pemphigus should be performed, including ACE inhibitors, D-penicillamine, angiotensin II receptor blockers, betablockers, cephalosporins, phenylbutazone, pyritinol, and tiopronin.
To decide on the best treatment regimen in FS, it is recommended to determine the extent of the disease. A practical approach is to assess the body surface area with active FS lesions, in which mild disease affects up to 1%, moderate disease up to 10%, and severe disease over 10% of the skin area, with 1% representing the sum of FS lesion areas that correspond approximately to the palmar area of the hands. Some dermatology departments have protocols to verify the extent and severity of the lesions using the Pemphigus Area and Activity Score (PAAS) and Autoimmune Bullous Skin Disorder Intensity Score (ABSIS).69,70
Before treatment begins, a complete blood count, creatinine, serum sodium and potassium, alanine transaminase (ALT), aspartate transaminase (AST), gamma glutamyl transferase, alkaline phosphatase, serum proteins, blood glucose, hepatitis B and C serology, HIV serology, and chest radiography should be ordered. Additional recommendations are to rule out IgA deficiency before administering IV immunoglobulin; thiopurine methyltransferase enzyme activity before azathioprine; abdominal ultrasound (optional), tuberculin skin test, or quantiferon-TB if there is high risk of TB; glucose-6-phosphate dehydrogenase deficiency, bilirubin levels, and reticulocyte parameters before dapsone; and β -HCG test to rule out pregnancy, bone mineral density before corticosteroid therapy, and ophthalmological evaluation to rule out glaucoma and cataract. Besides coproparasitology, prophylaxis of strongyloidiasis is recommended, as well as antibiotic therapy whenever pyoderma is present.
In localized forms with a limited number of lesions (up to 1% of body surface area), topical corticosteroids (from moderate to high potency), intralesional corticosteroids (triamcinolone acetonide 2-3mg/ml) or calcineurin inhibitors are used. Associated with topical therapy, dapsone 50-100mg a day may be prescribed. In some patients, low-dose prednisone (0.25mg/kg/d) can be prescribed.
Systemic corticosteroid (prednisone/prednisolone) is prescribed at a dose of 0.5mg/kg/day whenever topical treatment is insufficient to control disease activity, or if there is worsening of the skin condition with an increase in the number of lesions. In severe disseminated forms, the dose is 1mg/kg/day of prednisone or prednisolone. Systemic corticosteroid therapy is still the most widely used and recognized treatment option. Equivalent doses of triamcinolone may be administered in FS-resistant patients.
Considering that prolonged therapy with high-dose systemic corticosteroids can lead to serious or even fatal adverse effects, the early association of corticosteroid-sparing drugs (adjuvants) such as methotrexate, azathioprine, and mycophenolate mofetil is important in patients with disseminated disease. According to recent publications, the best combination would be deflazacort with azathioprine. Among the factors to consider in the choice of an adjuvant therapy are the availability, cost, and side effects of the medication.
Immunosuppressants (first-line adjuvant therapy):
Optimal adjuvants are azathioprine and mycophenolate mofetil, which have known corticoid-sparing effects. Azathioprine (AZA) is used at 1-3mg/kg/d, starting at 50mg/day and escalating gradually; whenever possible, thiopurine-methyl transferase activity should be verified before initiating treatment, because low levels of the medication can compromise the bone marrow. Mycophenolate mofetil (MFM) or mycophenolic acid is given at a dose of 2g/d, starting at 1g/d and increasing 500mg/week to improve gastric tolerance, providing an excellent but expensive option.
Methotrexate (MTX) is an interesting option due to its low cost and easy availability, but it is hepatotoxic. It is used at a dose of 7.5 to 25mg/week on 1 day or 2 consecutive days. After 24 hours, folic acid at a dose of 5mg must be prescribed. Alcohol, sulfonamides, and allopurinol must not be used concurrently.
For mild forms, dapsone (DDS)100mg/d or up to 1.5mg/kg/d can be used, since it also has a corticosteroid-sparing effect; however, glucose-6-phosphate dehydrogenase (G6PD) activity should be verified first. Considering that pemphigus is an antibody-mediated disease, the use of dapsone is controversial. According to study by a group from Philadelphia, USA, dapsone is ineffective in this setting.
Cyclophosphamide at a dose of 500mg IV bolus or 2 mg/kg/d has a corticosteroid-sparing effect, but the possibility of sterility, hemorrhagic cystitis, and secondary malignant neoplasm should be considered.
Newer Relevant TherapiesRituximab (anti-CD20 monoclonal antibody) is indicated when the patient shows previous treatment resistance or if prednisone is required at doses greater than 100mg/d associated with immunosuppressive therapy for more than 6 months. It has been used as a unit of pulse therapy at 1g IV, repeated at 15 days (rheumatoid arthritis protocol), or even 375mg/m2/weekly in four sessions (lymphoma protocol). Lower doses are ineffective. If necessary, the treatment can be repeated after 6 months. It can be combined with prednisone, in a regression regimen of up to 4 months or immunosuppressive regimen of up to 12 months. Hypersensitivity to murine proteins should be ruled out. Adverse effects include infections (about 10%) and rarely Stevens- Johnson syndrome and progressive multifocal leukoencephalopathy have been reported (the latter is a potentially fatal complication). Some specialized centers are using rituximab therapy in all PV and PF patients (initial and maintenance).
Intravenous immunoglobulin (IVIg) is also indicated in patients with severe treatment resistance or those experiencing significant adverse effects. It has been used initially in severe, disseminated cases of pemphigus because the clinical response appears to be faster. Doses of 2 to 3 g/kg/cycle are recommended (cycle of 4 to 5 consecutive days), every 30 days. Systemic corticosteroids and adjuvant drugs are maintained, which have been used in combination with rituximab. As a rare side effect, aseptic meningitis has been reported. IgA deficiency should be ruled out before starting this treatment.
Future Therapies:Immunoadsorption is a selective extracorporeal clearance technique which allows the removal of immunoglobulins, especially IgG1, IgG2, and IgG4. The technique can lead to reductions greater than 80% in circulating immunoglobulin levels. It is an additional option for refractory or very severe patients, available in advanced centers for the treatment of autoimmune diseases. Monthly cycles of 4 consecutive days are performed, with 2.5 times the plasma volume per day. Contraindications include severe systemic infection, severe cardiovascular disease, extensive hemorrhagic diathesis, and use of ACE inhibitors.
Figure 8shows an algorithm for treatment of FS.
Clinical and Laboratory Follow-upMaintenance after the consolidation phase: progressive corticosteroid (CS) reduction after disease control or by the end of the consolidation phase should be established, decreasing the CS dose by around 25% every 2 weeks until the dose of 20 mg per day is reached, after which the CS dose can be tapered further. If more than three lesions appear, the previous dose should be applied. When relapse occurs, the dose of the two previous phases should be applied. If the cutaneous lesions fail to stabilize within 2 weeks, the initial dose should be prescribed. In case the therapy is already combined with an immunosuppressant, the latter should be replaced, or IVIg, immunoadsorption, or rituximab should be used. High levels of anti-Dsg1 ELISA indicate the possibility of cutaneous relapses.
Clinical and laboratory follow-up: Clinical reassessment of the skin and mucous membranes should be performed every 2 weeks and then monthly after the disease is controlled. The following adverse effects should be investigated: diabetes mellitus, systemic arterial hypertension, and cardiac failure due to steroid therapy; respiratory distress, anemia, and hepatitis due to DDS and MTX; respiratory infections and hepatitis due to CS and immunosuppressants; mental disorders due to CS; myopathy, osteoporosis, avascular bone necrosis, glaucoma, and cataract due to CS; and hematological abnormalities due to immunosuppressive therapy.
Vaccination: The use of adjuvant immunosuppressants and rituximab contraindicate live virus vaccination.
Serological monitoring: serology (IIF and/or ELISA) should be performed at the start of treatment, at 3 months, and then according to evolution of the disease.
Discontinuation of treatment: primarily based on clinical signs, it can be monitored by anti-Dsg ELISA and IIF. Some dermatology departments recommend that the DIF should be negative. CS should be discontinued in patients with complete remission and minimal therapy, and the same process should be applied with adjuvants 6 to 12 months later.
Questions1. Pemphigus is defined as:
a) Cutaneous vesiculobullous lesions with subepidermal acantholysis and the presence of anti-desmoglein antibodies.
b) Intraepidermal and/or intraepithelial acantholytic vesiculobullous lesions with IgG4 antibodies.
c) Acantholytic vesiculobullous lesions with the presence of antibodies against the intercellular space of the epidermis/epithelium.
d) Non-acantholytic vesiculobullous lesions, Nikolsky’s sign, and IgG4 antibodies.
2. FS ecological systems share similarities with those of:
a) Chagas disease and leishmaniasis.
b) Leishmaniasis and yellow fever.
c) Lyme disease and yellow fever.
d) Lyme disease and Chagas disease.
3. Laboratory evidence that FS can be triggered by hematophagous insect bites:
a) Anti-Dsg1 monoclonal autoantibodies from FS patients react to the LJM11 protein.
b) Predominance of the black fly Simulium pruinosum in endemic areas of FS.
c) Passive immunization in mice with Lutzomya longipalpis saliva antigen produces anti-Dsg1 antibodies.
d) High IgE in the majority of FS patients.
4. The most common drugs that can precipitate pemphigus foliaceus are:
a) Penicillin and sulfonamides.
b) Nonsteroidal anti-inflammatory drugs and sulfonamides.
c) D-penicillamine and captopril.
d) Phenytoin and rifampicin.
5. In relation to FS autoantibodies:
a) The autoantibodies are more concentrated in the upper layers of the epidermis irrespective of the type of Dsg.
b) In endemic areas, normal individuals may present IgG4 autoantibodies.
c) Both IgG1 and IgG4 present high levels in FS patients with active disease.
d) Autoantibodies, being extracellular, do not require intracellular signaling mechanisms to trigger acantholysis.
6. For Senear-Usher syndrome, which of the following is FALSE:
a) The syndrome is a mild form of pemphigus foliaceus.
b) It is synonymous with pemphigus erythematosus.
c) It applies to patients with clinical-laboratory abnormalities of foliaceus pemphigus and lupus erythematosus.
d) Because it is misinterpreted, some authors suggest abandoning this nomenclature.
7. A 28-year-old female patient from the interior of Mato Grosso do Sul State, Brazil, presents with erythematous-crusty plaques on the scalp. To confirm endemic pemphigus foliaceus in this patient, it is sufficient to:
a) Verify whether the epidemiology is consistent with FS.
b) Identify subcorneal acantholytic cleavage and good response to systemic steroid therapy.
c) Show histopathology consistent with pemphigus foliaceus and IgG deposits in the intercellular space of the epidermis by indirect immunofluorescence.
d) Show IgG deposits in the intercellular space of the epidermis by direct immunofluorescence of biopsied skin fragment adjacent to the lesion.
8. During follow-up, the patient from the previous question may:
a) Present lesions on the oral mucosa.
b) Never evolve to erythroderma.
c) Present tense blisters as the disease spreads.
d) Present urticariform plaques with vesicles, and on histopathology, eosinophilic spongiosis with subcorneal pustules.
9. If the patient from the previous question becomes pregnant, her newborn: Has high probability of presenting neonatal fogo selvagem.
b) If the mother had pemphigus vulgaris, the infant would be less likely to develop neonatal pemphigus.
c) If neonatal pemphigus develops, this form may become chronic.
d) The infant rarely presents neonatal pemphigus unless the mother has disseminated disease and high autoantibody titers at the time of delivery.
10. Regarding immunological tests in FS:
a) Indirect immunofluorescence has high sensitivity and specificity.
b) Direct immunofluorescence is the gold standard and is performed on a biopsy of apparently normal skin close to the lesion.
c) Recombinant desmoglein ELISA has high sensitivity and lower specificity than indirect immunofluorescence.
d) All of the above are true.
Answers
Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93(4):495-506.
1. D
2. B
3. C
4. B
5. C
6. C
7. A
8. C
9. A
10. C
Papers
Information for all members: The EMC-D questionnaire is now available at the homepage of the Brazilian Annals of Dermatology: www.anaisdedermatologia.org.br. The deadline for completing the questionnaire is 30 days from the date of online publication.
Financial support: None.
Conflict of interest: None.