Dear Editor,
The development of immunobiological drugs in recent decades has led to major advances in the treatment of psoriasis and psoriatic arthritis. Drugs such as anti-tumor necrosis factor (anti-T-NF) and anti-interleukin 12/23 (anti-IL 12/23), for instance, have proven to be safe and effective alternatives, despite their high production cost.1
Biosimilars are immunobiological drugs that are similar to the original immunobiological product, manufactured using a cell line similar to that of the originally marketed drug.2 In order for bio-similars to be authorized and marketed, the patent on the original drug must expire first.1
The decrease in manufacturing costs and the possibility of expanding treatment to a larger share of the population make biosimilars an attractive alternative, although most regulatory agencies have approved them for psoriasis and psoriatic arthritis as an extrapolation of the efficacy confirmed in studies on other diseases, such as rheumatoid arthritis and ankylosing spondylitis.
CT-P13, an infliximab biosimilar, was authorized by the European Medicines Agency (EMA) in 2013 and the U.S. Food and Drug Administration (FDA) in 2016.3,4 In Brazil, CT-P13 was approved by the National Health Surveillance Agency (ANVISA) in 2016 based on a comparability exercise, thus becoming the first biosimilar drug for the treatment of psoriasis in the country.
Considering that data on efficacy, safety, and immunogenicity of biosimilars for psoriasis and psoriatic arthritis are still limited, replacing an original drug with its biosimilar remains an important matter of debate. In 2017, the Health Department of Brazil’s Federal District demanded that the original infliximab (Remicade®) be substituted by a biosimilar (Remsima®) in both treatment-naïve patients and those already in treatment.
This article reports our experience with the infliximab biosimilar in a 1-year follow-up study.
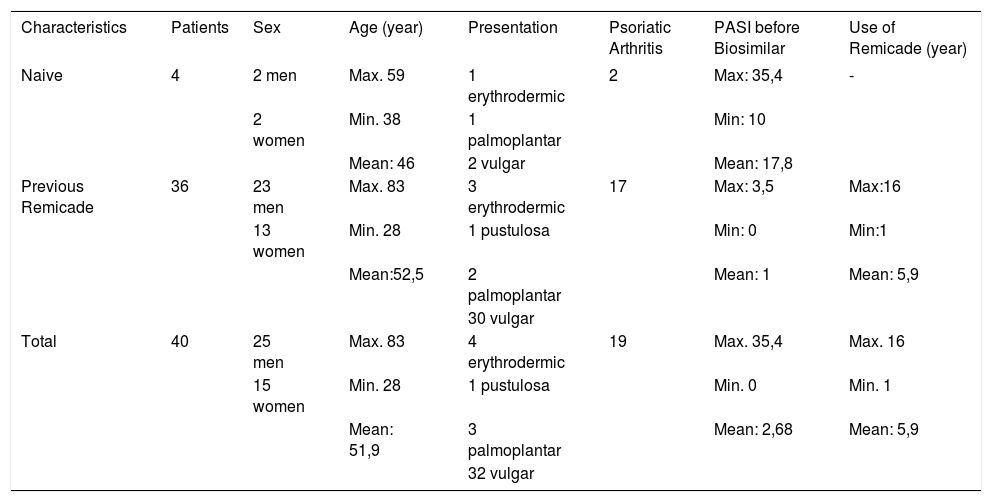
The study included 40 psoriasis patients who required or were already treated with infliximab, followed at the Brasilia University Hospital or at the Asa Norte Regional Hospital (Table 1).
Patients’ Profile
| Characteristics | Patients | Sex | Age (year) | Presentation | Psoriatic Arthritis | PASI before Biosimilar | Use of Remicade (year) |
|---|---|---|---|---|---|---|---|
| Naive | 4 | 2 men | Max. 59 | 1 erythrodermic | 2 | Max: 35,4 | - |
| 2 women | Min. 38 | 1 palmoplantar | Min: 10 | ||||
| Mean: 46 | 2 vulgar | Mean: 17,8 | |||||
| Previous Remicade | 36 | 23 men | Max. 83 | 3 erythrodermic | 17 | Max: 3,5 | Max:16 |
| 13 women | Min. 28 | 1 pustulosa | Min: 0 | Min:1 | |||
| Mean:52,5 | 2 palmoplantar | Mean: 1 | Mean: 5,9 | ||||
| 30 vulgar | |||||||
| Total | 40 | 25 men | Max. 83 | 4 erythrodermic | 19 | Max. 35,4 | Max. 16 |
| 15 women | Min. 28 | 1 pustulosa | Min. 0 | Min. 1 | |||
| Mean: 51,9 | 3 palmoplantar | Mean: 2,68 | Mean: 5,9 | ||||
| 32 vulgar |
After the biosimilar was introduced, all participants were followed for 1 year. During this time, two patients reported mild worsening of their skin lesions, but no significant increase in the PASI score was observed. Two patients developed infliximab-related infusion reactions. One was an infliximab-naïve patient who had a reaction during the second administration of the biosimilar drug (Remsima). The other had been using the original infliximab since 2015, but had stopped treatment 4 months earlier due to shortage of the medication, developing an infusion reaction during the first dose of Remsima. In addition, one infliximab-naïve patient lost weight, had a strong reaction to the PPD test, and developed pulmonary nodules after 7 months of treatment with the biosimilar. When the diagnosis of pulmonary tuberculosis was confirmed, the medication was discontinued and treatment for tuberculosis was initiated.
The 35 remaining patients had no reactions or adverse effects and showed controlled skin and/or joint symptoms, with PASI scores ranging from 0 to 2.7.
CT-P13 (Remsima®, Pfizer) is biologically similar to the original infliximab, a chimeric human-murine monoclonal antibody to TNF-α.3 It has been commercially available in Brazil since 2017, after the patent of the original drug had expired, and it was approved for the same indications as the original drug, including rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, rectal ulcerative colitis, psoriatic arthritis, and psoriasis.
Like previous European studies, our results suggest that the introduction or the switch to CT-P13 appears to be safe. Regarding drug efficacy, the original and the biosimilar showed similar results in most patients. 4,5 It is important to highlight that despite these positive results, most of our patients were clinically stable at the beginning of the study, showing good previous control.
Studies with larger cohorts and longer follow-up are still needed, but our preliminary results show that biosimilars may provide an opportunity to reduce health system costs because of their similarity to reference drugs in terms of safety and efficacy.