Background: Contact dermatitis affects up to 20% of the population. Patch testing for contact allergy may be needed to confirm the diagnosis.
Objectives: To describe and discuss the results of patch tests performed in a city in southern Brazil.
Methods: A cross-sectional analysis was performed on all skin test results over ten years. Variables such as gender, age at the time of testing, and test results were evaluated. Triggering factors, duration of complain, and previous medications used related to the clinical history were retrieved for some patients by reviewing their medical records.
Results: The sample was composed of 539 patch tests, of which 411 (76.2%) were from women. The age of the tested subjects ranged from 5 to 87 years. The prevalence of positive reactions in the patch tests was 391 (72.5%). The most prevalent positive reaction was to nickel sulfate (196; 36.4%), which had statistical significance when associated with female gender (p<0,001).
Study limitations: Database obtained through secondary sources (the reports of the exams and the medical records), occurring the incomplete registration of some information.
Conclusions: Data analysis at the local level is important to define preventive policies.
Contact dermatitis (CD) is a universal and common disease that affects 15% to 20% of the population sometime during life, standing out among occupational diseases. Regarding sex, females are twice as common. In Europe, nearly 20% of the general population suffers from contact allergy to at least one allergen.1 It constitutes the third cause of dermatology consultation in the United States and, in the year of 2004, affected 72 million people, leading to 9.2 million dermatology consultations.2 CD occurs in all ethnicities (lower incidence in dark skinned people) and ages, having had a marked increase during childhood as shown in recent studies.3,4
The prevalence of CD is uneven between populations due to different antigen exposures in each region. There are more than 3,700 substances that can cause it. In broad terms, CD depends on the time of exposure, frequency and the sensitizing potential of the antigens.3
CD is manifested more frequently as eczema, characterized by pruritus, erythematous, papular and vesicular lesions, and lichenification.1
Allergic contact dermatitis (ACD) is characterized by two distinct phases: the afferent phase, which corresponds to sensitizations, and the efferent phase, which represents elicitation. In the afferent phase, the first contact of the skin with the hapten and the replication of hapten-specific T-cells in lymph nodes take place. In the efferent phase, which happens some hours after repeat exposure to the hapten, there is elicitation of the production of chemokines, activation of endothelial cells and mast cells and infiltration of neutrophils, all required for the recruitment of specific T-cells.5-7
The diagnosis of ACD is achieved by specialized history-taking and physical examination, both of which can be complemented with patch tests. Also known as epicutaneous test, it is the gold-standard to help in the diagnosis. Its aim is to demonstrate hypersensitivity immunological contact reactions directly in the patient’s skin, therefore considered as an in vivo biological test.5 The presence of a positive test associated to a relevant clinical history confirms the diagnosis of ACD.8
When positive, lesions on the tested area develop, and these areas are classified according to the severity of the dermatitis, interpreted as 1 (+), 2 (++) and 3 (+++). The test is usually performed with the application of a standard series with 30 substances (Brazilian). The first reading is conducted after 48h and the second after 96h of the first contact with the allergens.9 The reading is based in the inspection and palpation of the test area, suggesting that the assessment is subject to the knowledge and experience of the reader.10, 11
Metals are among the main allergens causing ACD, among which nickel, cobalt and chrome are highlighted for having significant importance due to being present in cleaning products, for example, and being potent triggers or maintaining the dermatitis. Nickel can also be related to the use of jewelry and piercings.12
Topical medications are also the cause for allergic contact dermatitis, many times associated to self-medication and iatrogenesis. The etiologic agents can be either the active substance or the ingredients (preservatives, acidulants, emulsifiers), many also found in cosmetics.13
MethodsA cross-sectional study was performed. The collection was conducted in the only site that provides contact tests in a municipality in the south of Santa Catarina, Brazil. The sample was made by convenience by all patch test reports performed from January 2004 to January 2013, with a total of 9 full years.
The patch test was performed as indicated, with readings after 48 and 96 hours of the first contact with the allergens (standard series of 30 substances – Brazilian), with the physical examination of the patient’s skin exposed to the test. The final sample constituted of 539 test reports, from which a questionnaire developed by the authors was completed.
The variables searched included the archived report of the tests performed, with information about the sex, age and substance positivity, and also when the patient was seen at the practice before the test was performed. The clinical information was complemented with the assessment of the medical records. Data related to clinical history and suspicious substances for the contact dermatitis were searched in the electronic records. We emphasize that there no repeat information/reports, meaning that the number of reports corresponds to the number of patients.
The study was approved by the Ethics Committee of the Universidade do Sul de Santa Catarina (CEP-UNISUL) with the number 25197313.9.0000.5369. Data were stored and analyzed with Epi Info version 3.5.4 and Statistical Package for the Social Sciences (SPSS) version 18.0. The association between variables of interest was analyzed through specific tests, such as Student’s T test for quantitative variables and chi-square or Fisher’s exact test for qualitative variables, with a significance level of 5%.
ResultsThe sample comprised 539 reports. Of this total, 411 (76.2%) were females.
The age when the test was performed ranged from 5 to 87 years, with a mean of 38.8, mode of 32 and median of 38. This variable was re-categorized according to the median.
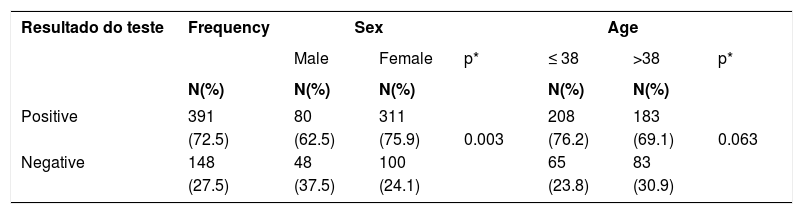
Three hundred and ninety one (72.5%) tests with positive results were found. Data regarding the association of the contact test results with the sex and re-categorized age are shown in table 1. Females had a higher percentage (75.9%) than males (62.5%).
Distribution of the sample according to the association of patch test results performed from 2004 to 2013 with grouped sex and age. City in the south of Santa Catarina, 2014 (n=539)
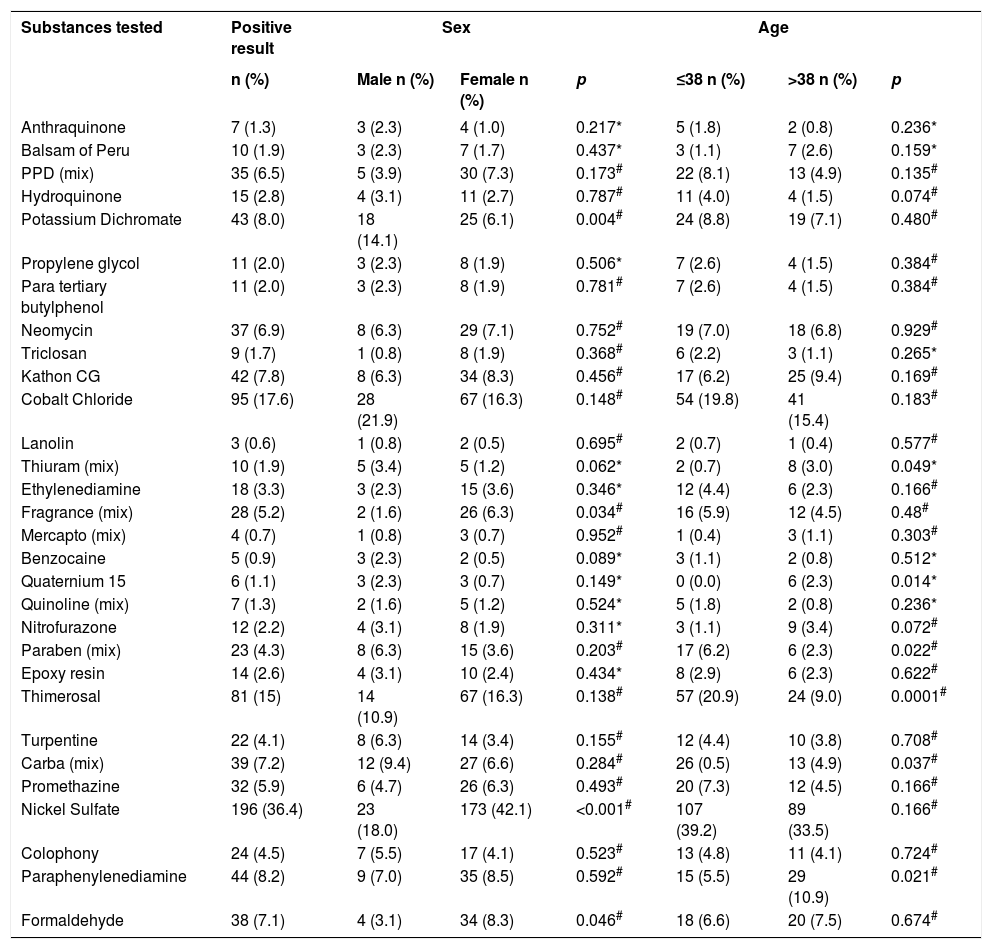
Nickel sulfate was the most prevalent positive substance, with positive results in 196 (36.4%) tests. Table 2 described the frequency of the 30 substances tested with positive results associated to re-categorized age and sex. Persons up to 38 years of age had more positive results to Paraben (mix), Thimerosal and Carba (mix), while with Thiuram (mix), Quaternium 15 and Paraphenylenediamine the most frequent positive results were for persons older than 38 years of age. Females had positive results to Potassium Dichromate and Nickel Sulfate, with lower and higher frequency than males, respectively.
Substances tested and their positive results associated to grouped sex and age, from 2004 to 2013 (n=539). City in the south of Santa Catarina, 2014
| Substances tested | Positive result | Sex | Age | ||||
|---|---|---|---|---|---|---|---|
| n (%) | Male n (%) | Female n (%) | p | ≤38 n (%) | >38 n (%) | p | |
| Anthraquinone | 7 (1.3) | 3 (2.3) | 4 (1.0) | 0.217* | 5 (1.8) | 2 (0.8) | 0.236* |
| Balsam of Peru | 10 (1.9) | 3 (2.3) | 7 (1.7) | 0.437* | 3 (1.1) | 7 (2.6) | 0.159* |
| PPD (mix) | 35 (6.5) | 5 (3.9) | 30 (7.3) | 0.173# | 22 (8.1) | 13 (4.9) | 0.135# |
| Hydroquinone | 15 (2.8) | 4 (3.1) | 11 (2.7) | 0.787# | 11 (4.0) | 4 (1.5) | 0.074# |
| Potassium Dichromate | 43 (8.0) | 18 (14.1) | 25 (6.1) | 0.004# | 24 (8.8) | 19 (7.1) | 0.480# |
| Propylene glycol | 11 (2.0) | 3 (2.3) | 8 (1.9) | 0.506* | 7 (2.6) | 4 (1.5) | 0.384# |
| Para tertiary butylphenol | 11 (2.0) | 3 (2.3) | 8 (1.9) | 0.781# | 7 (2.6) | 4 (1.5) | 0.384# |
| Neomycin | 37 (6.9) | 8 (6.3) | 29 (7.1) | 0.752# | 19 (7.0) | 18 (6.8) | 0.929# |
| Triclosan | 9 (1.7) | 1 (0.8) | 8 (1.9) | 0.368# | 6 (2.2) | 3 (1.1) | 0.265* |
| Kathon CG | 42 (7.8) | 8 (6.3) | 34 (8.3) | 0.456# | 17 (6.2) | 25 (9.4) | 0.169# |
| Cobalt Chloride | 95 (17.6) | 28 (21.9) | 67 (16.3) | 0.148# | 54 (19.8) | 41 (15.4) | 0.183# |
| Lanolin | 3 (0.6) | 1 (0.8) | 2 (0.5) | 0.695# | 2 (0.7) | 1 (0.4) | 0.577# |
| Thiuram (mix) | 10 (1.9) | 5 (3.4) | 5 (1.2) | 0.062* | 2 (0.7) | 8 (3.0) | 0.049* |
| Ethylenediamine | 18 (3.3) | 3 (2.3) | 15 (3.6) | 0.346* | 12 (4.4) | 6 (2.3) | 0.166# |
| Fragrance (mix) | 28 (5.2) | 2 (1.6) | 26 (6.3) | 0.034# | 16 (5.9) | 12 (4.5) | 0.48# |
| Mercapto (mix) | 4 (0.7) | 1 (0.8) | 3 (0.7) | 0.952# | 1 (0.4) | 3 (1.1) | 0.303# |
| Benzocaine | 5 (0.9) | 3 (2.3) | 2 (0.5) | 0.089* | 3 (1.1) | 2 (0.8) | 0.512* |
| Quaternium 15 | 6 (1.1) | 3 (2.3) | 3 (0.7) | 0.149* | 0 (0.0) | 6 (2.3) | 0.014* |
| Quinoline (mix) | 7 (1.3) | 2 (1.6) | 5 (1.2) | 0.524* | 5 (1.8) | 2 (0.8) | 0.236* |
| Nitrofurazone | 12 (2.2) | 4 (3.1) | 8 (1.9) | 0.311* | 3 (1.1) | 9 (3.4) | 0.072# |
| Paraben (mix) | 23 (4.3) | 8 (6.3) | 15 (3.6) | 0.203# | 17 (6.2) | 6 (2.3) | 0.022# |
| Epoxy resin | 14 (2.6) | 4 (3.1) | 10 (2.4) | 0.434* | 8 (2.9) | 6 (2.3) | 0.622# |
| Thimerosal | 81 (15) | 14 (10.9) | 67 (16.3) | 0.138# | 57 (20.9) | 24 (9.0) | 0.0001# |
| Turpentine | 22 (4.1) | 8 (6.3) | 14 (3.4) | 0.155# | 12 (4.4) | 10 (3.8) | 0.708# |
| Carba (mix) | 39 (7.2) | 12 (9.4) | 27 (6.6) | 0.284# | 26 (0.5) | 13 (4.9) | 0.037# |
| Promethazine | 32 (5.9) | 6 (4.7) | 26 (6.3) | 0.493# | 20 (7.3) | 12 (4.5) | 0.166# |
| Nickel Sulfate | 196 (36.4) | 23 (18.0) | 173 (42.1) | <0.001# | 107 (39.2) | 89 (33.5) | 0.166# |
| Colophony | 24 (4.5) | 7 (5.5) | 17 (4.1) | 0.523# | 13 (4.8) | 11 (4.1) | 0.724# |
| Paraphenylenediamine | 44 (8.2) | 9 (7.0) | 35 (8.5) | 0.592# | 15 (5.5) | 29 (10.9) | 0.021# |
| Formaldehyde | 38 (7.1) | 4 (3.1) | 34 (8.3) | 0.046# | 18 (6.6) | 20 (7.5) | 0.674# |
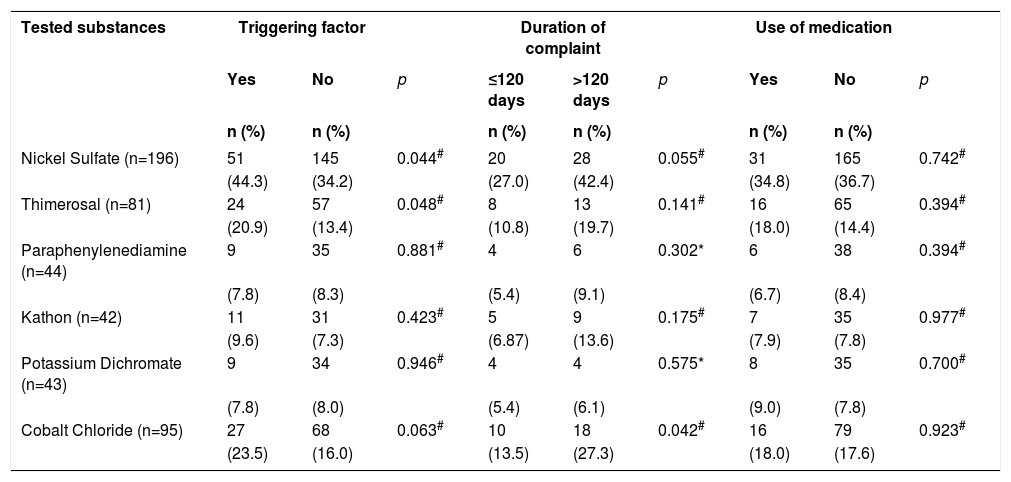
Clinical data as triggering factors, use of previous medications to the test and duration of the complaint were retrieved from the files of 200 (37.1%) patients. The duration of the complaint that lead to the test ranged from 1 to 7,300 days, with a median of 120 days.
The associations between the factors mentioned above and the main substances with positive results can be seen in table 3. Positivity for Cobalt Chloride was more frequent among those who reported complaints for longer than 120 days. For Nickel Sulfate and Thimerosal, those with a triggering factor had a higher percentage of positive results.
Association of the six most frequently positive substances and triggering factor, duration of complaint and use of medication prior to contact test, from 2004 to 2013 (n=539). City in the south of Santa Catarina, 2014
| Tested substances | Triggering factor | Duration of complaint | Use of medication | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p | ≤120 days | >120 days | p | Yes | No | p | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||
| Nickel Sulfate (n=196) | 51 | 145 | 0.044# | 20 | 28 | 0.055# | 31 | 165 | 0.742# |
| (44.3) | (34.2) | (27.0) | (42.4) | (34.8) | (36.7) | ||||
| Thimerosal (n=81) | 24 | 57 | 0.048# | 8 | 13 | 0.141# | 16 | 65 | 0.394# |
| (20.9) | (13.4) | (10.8) | (19.7) | (18.0) | (14.4) | ||||
| Paraphenylenediamine (n=44) | 9 | 35 | 0.881# | 4 | 6 | 0.302* | 6 | 38 | 0.394# |
| (7.8) | (8.3) | (5.4) | (9.1) | (6.7) | (8.4) | ||||
| Kathon (n=42) | 11 | 31 | 0.423# | 5 | 9 | 0.175# | 7 | 35 | 0.977# |
| (9.6) | (7.3) | (6.87) | (13.6) | (7.9) | (7.8) | ||||
| Potassium Dichromate (n=43) | 9 | 34 | 0.946# | 4 | 4 | 0.575* | 8 | 35 | 0.700# |
| (7.8) | (8.0) | (5.4) | (6.1) | (9.0) | (7.8) | ||||
| Cobalt Chloride (n=95) | 27 | 68 | 0.063# | 10 | 18 | 0.042# | 16 | 79 | 0.923# |
| (23.5) | (16.0) | (13.5) | (27.3) | (18.0) | (17.6) | ||||
In regard to patients who had the triggering factors described in the files (n=115), 40 (34.8%) worsened by using nail polish, 25 (21.7%) with jewelry accessories, 19 (16.5%) with cosmetics, 17 (14.8%) with latex, 14 (12.2%) with makeup, 8 (6.9%) with cement, 3 (2.6%) with lime, 3 (2.6%) with formaldehyde and 23 (20.0%) with “others” including cleaning products, handicraft, pesticides, flowers and leather. We highlight that of the topical and systemic medications used described in the patient’s files before the test was performed (n=89), 50 (56.8%) were corticosteroids, 30 (33.7%) were antihistamines, 13 (14.6%) were antifungals and 22 (24.7%) belonged to other groups which included moisturizers, antibiotics, anti-parasitic and oral retinoids. The emphasize that the same patient could have mentioned more than one triggering factor and used more than one class of medication.
Regarding the specialty of the requesting physician, 211 (39.1%) were requested by dermatologists, 219 (40.6%) by allergists, 10 (1.9%) by other specialties and 99 (18.4%) of the requests were not specified in the report.
DiscussionThe prevalence of positive contact tests in the sample studied was of 391 (72.5%). A study by Boonchai and Iamtharachai et al showed 692 (81.2%) positive tests, with results that are relatively close. The present study shows similarities regarding the sample of that mentioned, for both were conducted in a period of ten years and test results and patient data were collected by reviewing medical records.14
As in the study by Rodrigues et al, the present study found a higher prevalence of females undergoing the 411 (76.2%).15 We can infer from this datum two possibilities, females seek medical attention more frequently and also females have a higher prevalence of contact dermatitis due to a higher exposure to allergens.1
We also observed a higher positivity of the tests among females, corresponding to 311 (75.9%) cases when compared to the positivity in males, that represented 80 (62.5%) cases (p=0.003), emphasizing the likelihood of females having a higher prevalence of contact dermatitis, what motivates the pursuit of performing test.1,16
Regarding age, a mean of 38.8 and a median of 38 years was seen. The age group observed is similar to the consulted literature, probably due to a higher exposure to sensitizing agents over the years. This fact is supported by the lower prevalence of allergic contact dermatitis in childhood.3,14
Despite the positivity of the test being more prevalent among those aged ≤ 38 years (208; 76.2%), this was not statistically significant (p=0.063).
Contact tests can be indicated when there is clinical suspicion of ACD, for patients with other skin conditions that can be aggravated by ACD, patients with chronic eczema with no clear etiology and suspicion of occupational ACD.2 The objective of this study was not to evaluate possible occupational causes. However, we noticed that, depending on the substance tested, there was an association with the sex, what can be closely related to the occupation.
The most prevalent positive substances found in this study were, in descending order: Nickel Sulfate (36.4%), Cobalt Chloride (17.6%), Thimerosal (15.0%), Paraphenylenediamine (8.2%) and Potassium Dichromate (8.0%). These data are in accordance with another study, with Nickel Sulfate in first, and the other not necessarily in the same order of prevalence, except for Paraphenylenediamine, which was the 8th more prevalent substance.17
Nickel Sulfate, the most prevalent substance, was positive in 196 (36.4%) cases and was statistically significant when associated to females (p<0.001). It is suggested that it can be related to the high use of products that contain it, such as jewelry, piercings, cleaning products, eyeglasses, watches, buttons in pants and gold products. These products can be more frequently used by women, what explains higher sensitization among them. An example is the piercing of the earlobes for using earrings among females, that can happen at birth in some cultures.18,19
With the goal of minimizing these findings, the European Union imposed some restrictions to Nickel Sulfate in the composition of some products and achieved a reduction in nickel allergy in youngsters of many countries including Germany, Sweden and Denmark. For example, in Denmark, there was a reduction from 26.9% to 12.4% in the frequency of nickel allergy after this measure.1
Cobalt Chloride, the second most prevalent substance in this study (95 cases; 17.6%), is related to products such as nail polishes and hair dye. Besides, it is described that contact dermatitis by this substance is closely related to the presence of this metal associated to materials which also contain nickel. Such as Nickel Sulfate, it was highly prevalent, what could be associated to a higher prevalence of positive results in females. However, relationship. With sex was not statistically significant (p=0.148).12,19,20
The third most prevalent substance was Thimerosal (15.0%) and, when associated to the age group, was statistically significant for the group ≤ 38 years (57; 20.9%) with the value of p=0.0001. In the study by Duarte et al it was found as the second most prevalent substance, but with percentages similar to those of this study. We highlight its presence in medication and vaccine preservatives, contact lenses solutions and tattoo inks, what can favor the contact with this substance.17
Paraphenylenediamine was positive in 44 cases (8.2%). It was statistically significant regarding age >38 years, with value of p=0.021. It is present in leather products, nail polishes, photocopies, greases, leather reagents and fabrics (black, blue, brown), hair dyes and X ray fluids.
Potassium Dichromate, 43 (8.0%) cases, is present in the construction industry, as a component of cement. It is suggested that is it responsible for one of the most frequent occupational dermatoses, mainly in males – as demonstrated in the present study (p=0.004). Another study showed similar positivity to this study for this substance, however, with a higher participation of males in the sample. Construction workers have prolonged contact with this allergen, what could promote sensitization, since many times the personal protection equipment is not used adequately.21,22
Formaldehyde was the eighth most prevalent substance (7.1%), positive in 8.3% of females and 3.1% of males (p=0.046). We can suggest as an explanation the fact that it is present in hair straighteners an synthetic nail polishes more frequently used by women, but it is also present in plastics, paints, varnishes, textile industry and foundry industry. One study points to it being the most prevalent substance when only the cosmetic series is tested.15
In the present study, Fragrance Mix had higher positivity in females (p=0.034), as well as in the study by Waranya Boonchai and Pacharee Iamtharachai et al. This substance is the main indicator for perfume ACD and is part of many fragrances found in cosmetics. It is suggested that because women use more beauty and hygiene products, they would be more prone to sensitization.14,23
Quaternium 15 (6; 1.1%) was one of the substances with less positive results, all of them among persons >38 years of age, with a prevalence of 2.3% in this population (p=0.014). It is found in shampoos, conditioners, liquid soaps, shaving products, moisturizing lotions, cosmetic, makeup products, sunscreens, topical medications, cleaning products, disinfectants and soaps.
Paraben Mix had more positive results in persons aged ≤ 38 years (6.2% versus 2.3% in hose >38 years) with p=0.022. This substance is used as a preservative in cosmetics, pharmaceutical products and also some foods, products that are potentially more used among younger people.
ConclusionThe prevalence of positive contact tests was of 72.5%, and among those 75.9% were females.
The five most prevalent substances in the sample were, in descending order, Nickel Sulfate (36.4%), Cobalt Chloride (17.6%), Thimerosal (15.0%), Paraphenylenediamine (8.2%) and Potassium Dichromate (8.0%).
The association between positivity to the test and the sex was statistically significant for Nickel Sulfate and females (p<0.001). The same happened with Potassium Dichromate (p=0.004), Fragrance Mix (p=0.034) and Formaldehyde (p=0.046).
One of the limitations of the study was the fact that the database was obtained through secondary sources: test reports and patient’s files. Regarding the files, we emphasize the fact that some records were incomplete, with only 200 (37.1%) of the total being retrieved.
Data verification is relevant in view of possible preventive policies and legislations that ensure consumer and worker protection, since a significant number of persons affected by ACD is in full work capacity, what can lead to a reduction in productivity of the economically active population.