Dear Editor,
The primary assay recommended for identifying phototoxicity is the 3T3 Neutral Red Uptake Phototoxicity Test (3T3 NRU PT). This in vitro method uses a 3T3 cell line from BALB/c mouse embryos (BALB/c clone A31). 3T3 NRU PT compares compound cytotoxicity tested in the presence and in the absence of exposure to a non-cytotoxic dose of UVA light. Cytotoxicity is evaluated by neutral red uptake (NRU) 24 hours after treatment.1 Nevertheless, some trials showed that a positive phototoxic result in the 3T3NRUPT may not correlate with results in human volunteers. The discrepancy is probably linked to several factors.2 However, when we performed the 3T3NRUPT test, we encountered some difficulty in detaching Balb/c from the cultivation plate surface during the washing steps and the subsequent incubation in phosphate-buffered saline (PBS).
To improve cell attachment, we tested 4 different variations of 96-well plates – Nunc®, collagen-coated Nunc®, TPP®, and collagen-coated TPP – and 3 washing solutions – Earle’s Balanced Salt Solution (EBSS containing 1mg/ml of glucose), PBS, and PBS supplemented with glucose (PBS-Glu; 1mg/ml). EBSS and PBS differing in the composition of inorganic salts and presence of glucose are recommended by OECD 432. Balb/c (clone A31) from the European Collection of Authenticated Cell Cultures (ECACC), were grown in DMEM supplemented with fetal calf serum (FCS; 5%), newborn calf serum (5%), penicillin (0.1mg/ml), and streptomycin (100U/ml). Human keratinocyte cell lines (HaCaT) were obtained from CLS (Germany) and cultured in DMEM supplemented with FCS (10%), penicillin (0.1mg/ml), and streptomycin (100U/ml). The cultures were tested concurrently. Cells were cultured in a humidified atmosphere with CO2 (5%) at 37°C. Twenty-four hours after seeding, cells were washed twice with washing solution. The respective solution was then applied and immediately or after 10-40 min replaced with serum-free DMEM. After 24 hours, the quantity of viable cells was evaluated by NRU, as described previously.3
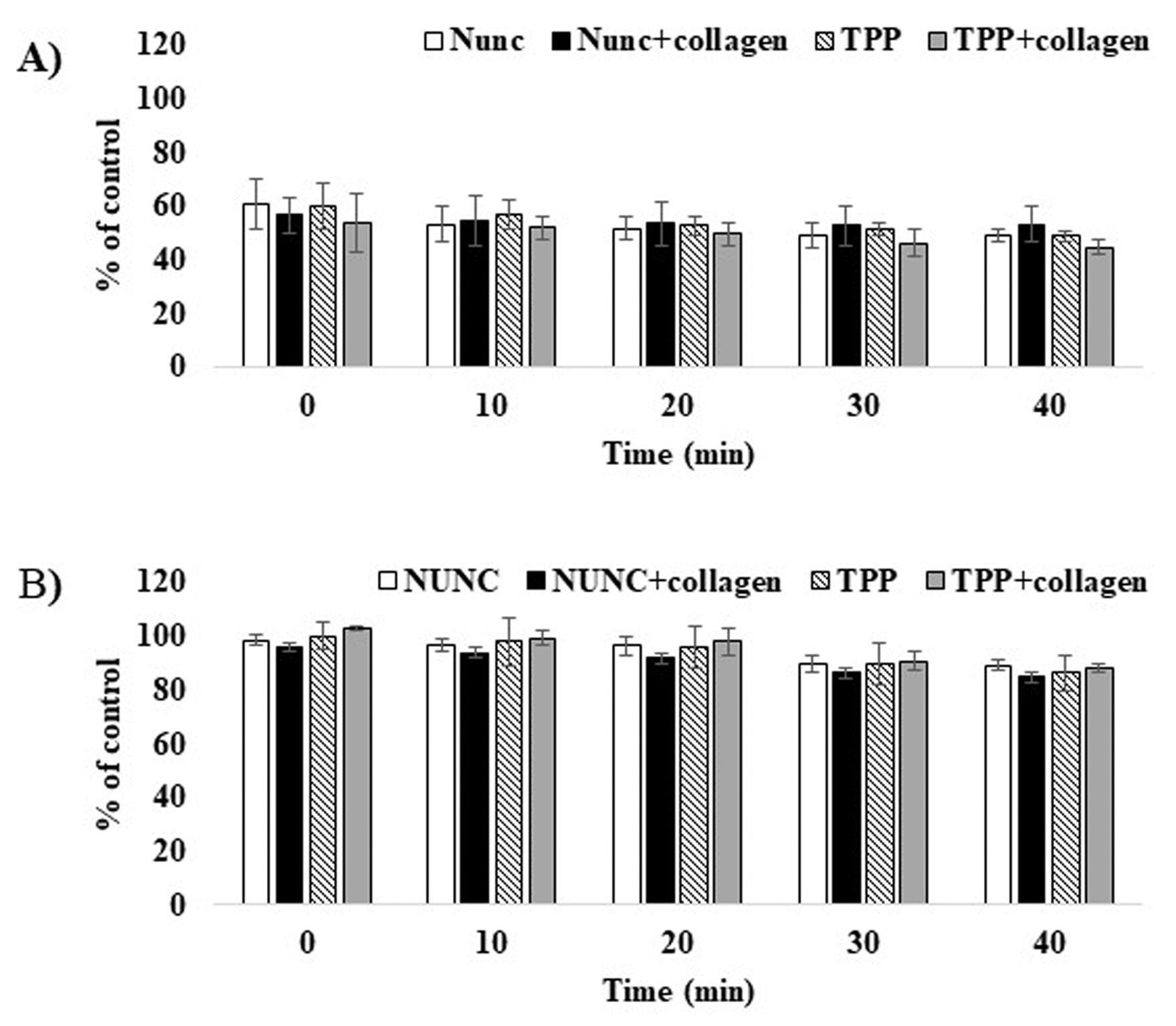
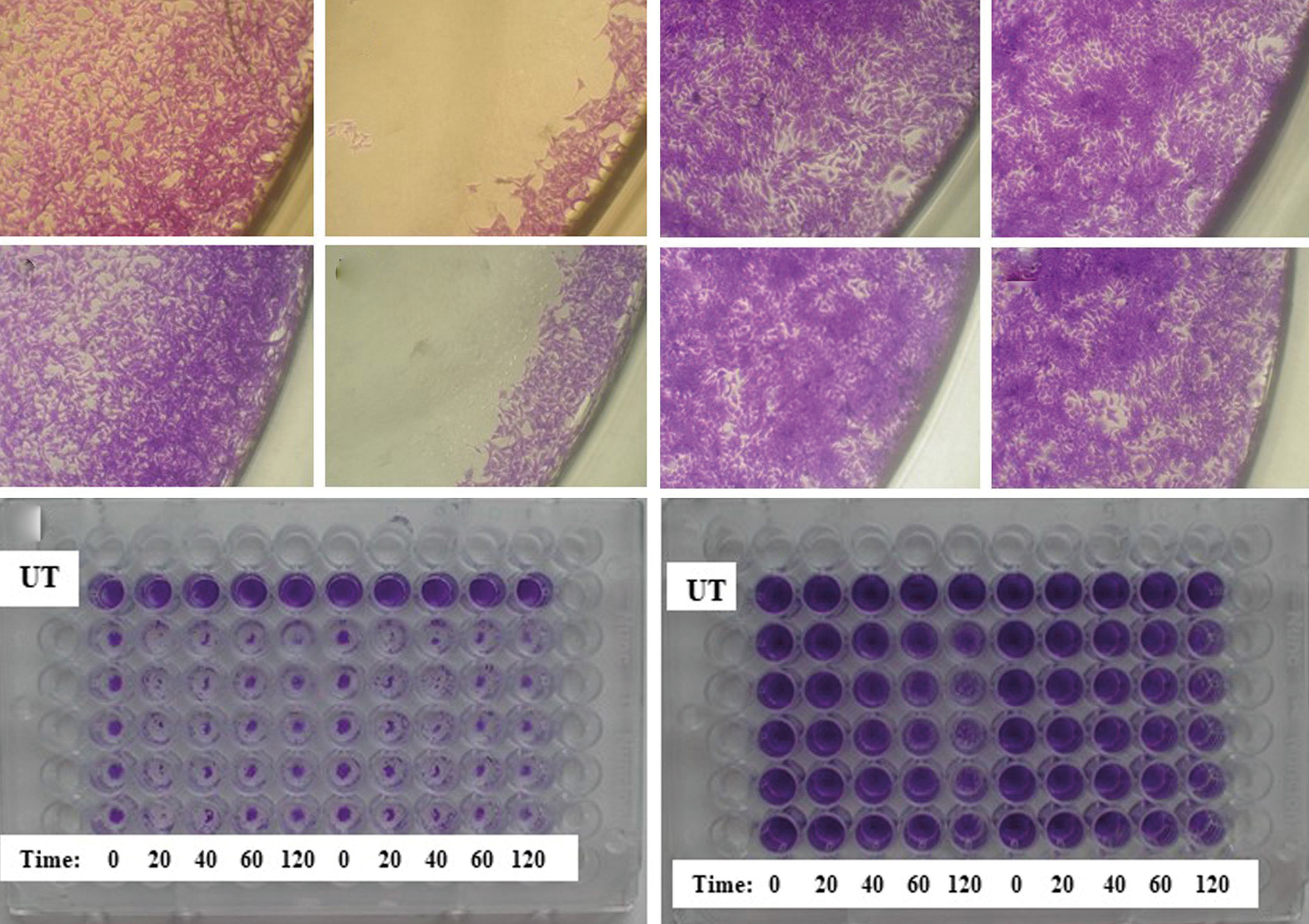
Results showed that Balb/c embryos were very susceptible to washing steps as the cell number (viability) decreased to ~60% of untreated cells (UTC). The decrease intensified with the duration of the incubation period in the washing buffer (only 45% of UTC). The expected advantageous effect on Balb/c attachment was not found in any combination of culture plate and buffered solution. Data for PBS are shown in Figure 1A. In contrast, minimal changes were found in HaCaT; the cell number was only reduced to 95% of UTC. After 40 min in individual solutions, the HaCaT number only slightly decreased to 90% of UTC. No significant effect on the cell number was found between the washing solutions and culture plate surfaces used. Data for PBS are shown in Figure 1B. Microscopic evaluation revealed that most of the remaining Balb/c were localized in the center and on the margin of the wells (Figure 2A-D). In contrast, the HaCaT cells were evenly spread across the whole bottom of the wells (Figure 2E-H). Further, in some Balb/c plates, the color intensity (cell number) significantly differed from others in the same time interval, and the difference in absorbance between the highest and the lowest intensity was 30-60%. This difference was caused by accidental cell detachment from the surface (demonstrated on fixed cells stained with crystal violet, Figure 2I). This significant Balb/c viability reduction may affect the results of phototoxicity evaluation. At least it could cause a problem in terms of meeting the acceptance criteria for the data obtained. According to OEDC 432, a test is acceptable if the mean absorbance at 540nm of NR extracted from untreated controls is higher than 0.4 (i.e. approximately 20 times the background solvent absorbance). In HaCaT, individual wells in the same time interval showed a comparable number of cells (Figure 2J). Minimal effects of the treatment on HaCaT is probably associated with the higher adherence of keratinocytes to the culture plate surface compared to fibroblasts. We had a similar experience with primary human dermal fibroblasts (HDF) and epidermal keratinocytes (HEK). While HDF are susceptible to detachment due to washing and incubation in PBS, a minimal effect is observed in HEK.2 It was also shown that activity of skin fibroblasts and keratinocytes adhesion proteins is influenced by the divalent cations Ca2+ and Mg2+ differently, which may contribute to the increased attachment of keratinocytes.4
Cell number (viability) after washing and PBS incubation procedures. Balb/c 3T3 A - and HaCaT B - cells were washed twice and incubated in PBS. Results are expressed as mean ± SD of % of control from 5 independent experiments. The statistical comparison was performed using Student’s t-test. Statistical significance was determined at p = 0.05
Morphology of Balb/c 3T3 and HaCaT. Representative examples of untreated Balb/c on A - Nunc® and C - TPP® plates. Balb/c washed twice and incubated for 40 min in PBS on B - Nunc® and D - TPP® plates. Representative examples of untreated HaCaT on E - Nunc® and G - TPP® plates. HaCaT washed twice and incubated for 40 min in PBS on F - Nunc® and H - TPP® plates. Representative example of I - Balb/c 3T3 and J - HaCaT on TPP® plate. After 2 washes with PBS and incubation for 0, 20, 40, 60 or 120 min in PBS, cells were incubated for 24 hours and then fixed with methanol and stained with crystal violet dye. UT = untreated cells
In conclusion, Balb/c have several advantages, e.g. availability, easy and cheap cultivation, and good reproducibility. However, HaCaT have similar attributes5 and come from skin cells of human origin. Recently, we have shown the capability of HaCaT to distinguish phototoxic and non-phototoxic compounds like Balb/c.2 Therefore, HaCaT seems to be at least a suitable alternative for phototoxicity screening alongside Balb/c, and both cell lines could be used concurrently.
AcknowledgementsFinancially supported by GACR 15-10897S.
Financial Support: This work was financially supported by the Grant Agency of the Czech Republic (GACR 15-10897S) and the Institutional Support of Palacký University in Olomouc RVO 61989592.
Conflict of interest: None.