Sentinel lymph node biopsy in thin invasive primary cutaneous melanoma (up to 1mm thick) is a controversial subject. The presence of tumor-infiltrating lymphocytes could be a factor to be considered in the decision to perform this procedure.
ObjectiveTo evaluate the association between the presence of tumor-infiltrating lymphocytes and lymph node metastases caused by thin primary cutaneous melanoma.
Methods:Cross-sectional study with 137 records of thin invasive primary cutaneous melanoma submitted to sentinel lymph node biopsy from 2003 to 2015. The clinical variables considered were age, sex and topography of the lesion. The histopathological variables assessed were: tumor-infiltrating lymphocytes, melanoma subtype, Breslow thickness, Clark levels, number of mitoses per mm², ulceration, regression and satellitosis. Univariate analyzes and logistic regression tests were performed as well the odds ratio and statistical relevance was considered when p <0.05.
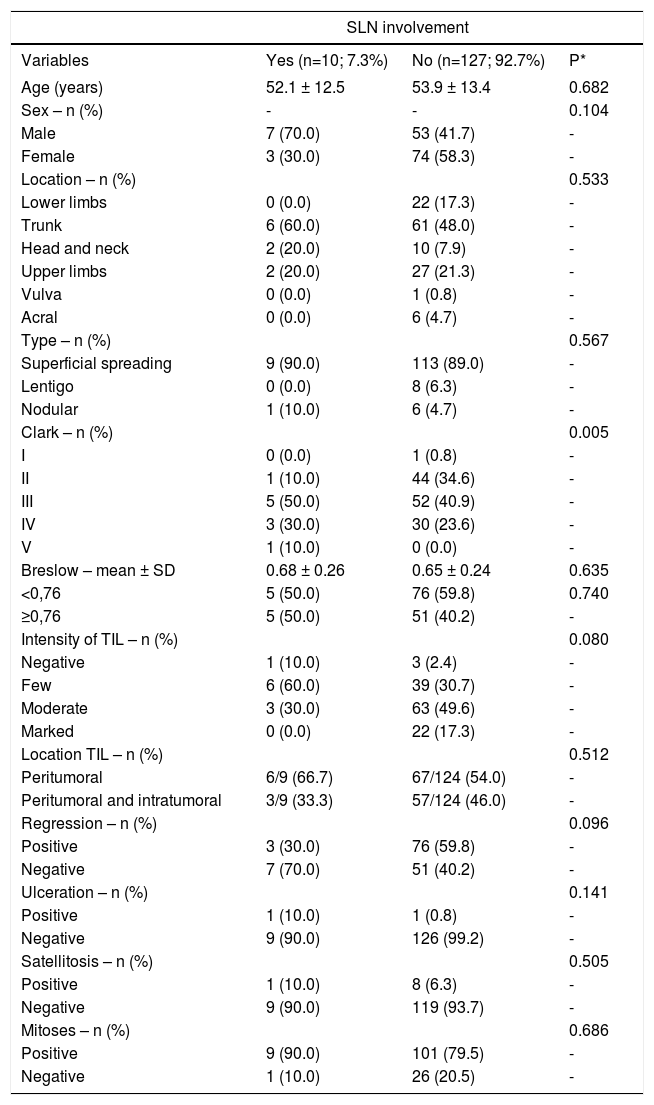
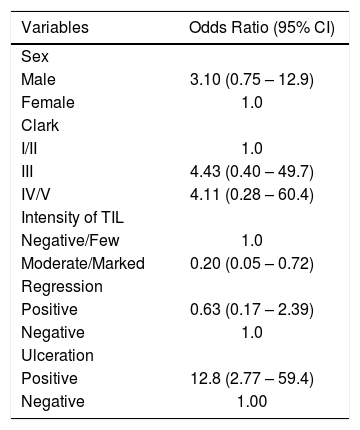
Results:Among the 137 cases of thin primary cutaneous melanoma submitted to sentinel lymph node biopsy, 10 (7.3%) had metastatic involvement. Ulceration on histopathology was positively associated with the presence of metastatic lymph node, with odds ratio =12.8 (2.77-59.4 95% CI, p=0.001). The presence of moderate/marked tumor-infiltrating lymphocytes was shown to be a protective factor for the presence of metastatic lymph node, with OR=0.20 (0.05-0.72 95% CI, p=0.014). The other variables - clinical and histopathological - were not associated with the outcome.
Study limitations:The relatively small number of positive sentinel lymph node biopsy may explain such an expressive association of ulceration with metastatization.
Conclusions:In patients with thin invasive primary cutaneous melanoma, few or absent tumor-infiltrating lymphocytes, as well as ulceration, represent independent risk factors for lymph node metastasis.
Early diagnosis of melanoma is a vital necessity in view of the reduced survival with the progression of the disease. In invasive early lesions, i.e., up to 1mm thickness and not in situ, lymph node involvement is one of the main prognostic factors.1,2 Thus, tumors classified as T1 (≤ 1mm thick) by the American Joint Committee on Cancer (AJCC), despite representing the majority of melanomas (63%), can belong to clinical stage III (due to lymph node involvement) and patients have their 5-year survival significantly reduced (98.9% for T1a and 93.7% for T1b) to 53.7%.3,4 This explains the fact that thin melanomas being responsible for up to 25% of death caused by this disease.3
The recommendation for sentinel lymph node biopsy (SLNB) in a thin cutaneous melanoma (≤ 1mm) is controversial, since some authors consider the chance of lymph node involvement to be low in this group, particularly when the thickness is of less than 0.75mm.5-10 On the other hand, some authors present data that add to the existing doubts on the subject. Fearfield et al. 11 found 2 to 18% of recurrence in up to 11 years when melanoma thickness was less than 0.76mm and Andtbacka & Gershenwald12 argued that 5.1% of positive lymph nodes (LN) in thin primary cutaneous melanomas (TPCM) would be enough to support the universalization of screening.
Tumor-infiltrating lymphocytes (TIL) play a central role in targeted-therapy revolution.13-15 Its understanding triggered advances in immunotherapy and their presence represents a favorable outcome with improved survival.16
The classification of the activity of TIL remained the same for approximately 30 years, since it was first categorized into brisk and non-brisk.17 A new classification was proposed with the addition of another level in lymphocytic activity, with standardization into few, moderate and marked. This new sub-classification was used in the study by Fortes.18,19
The main objective of this study was to investigate the relationship between metastatic involvement of sentinel lymph node and the presence and intensity of tumor-infiltrating lymphocytes. The secondary objectives were to compare the other clinical characteristics and pathological microstaging with the same outcome.
MethodsCross-sectional study conducted with data registered between 2003 and 2015 in the Laboratory of Pathology, Santa Casa de Porto Alegre, an oncology reference center in the south of Brazil. Cases with invasive lesions ≤1mm, with no clinical lymph node involvement, submitted to SLNB were eligible. Clinical and histological variables were assessed: age, sex, topography of the lesion, histological subtype, presence of TIL (graded into few, moderate and marked), Breslow thickness, Clark levels, mitoses, ulceration, regression and satellitosis. The study was approved by the committee of ethics in research of the institution under the number 653.823.
After the diagnosis of melanoma with excisional biopsy, the patients were submitted to staging with clinical examination and imaging studies. In the immediate pre-operative period, the patients were reassessed and only those cases with no lymph node involvement were included in the study, ruling out the possibility of over-indicating SLNB in view of an already existing indication for lymphadenectomy. All SLNB negative on examination with hematoxylin & eosin were submitted to immunohistochemistry. All patients diagnosed with secondary implants in the sentinel lymph nodes were submitted to lymphadenectomy.
For the statistical analysis, the level of significance adopted was 5% (p<0.05) and the analyses were made using the program SPSS version 21.0. The main outcome was metastatic involvement of the sentinel lymph node. The Odds Ratio (OR), together with the 95% confidence interval, was calculated to estimate the effect of each factor on the main outcome. The ROC (Receiver Operating Characteristic) curve was evaluated to estimate the best cut-off point for Breslow thickness according to sentinel lymph node positivity and the area on the curve was calculated to estimate the accuracy of this score for predicting the outcome. Student’s T test was used for the comparison of the means between patients with and without sentinel lymph node involvement. In case of asymmetry, the Mann-Whitney test was used. Fisher’s exact test or Pearson’s chi-square test (variables with more than two categories) were used in the comparison of proportions. Poisson regression was used for the multivariate analysis.
ResultsThe mean age of the patients in the study was 53 years, with a non-significant predominance of females. The trunk was the site most affected by melanoma and the most frequent type of melanoma was the superficial spreading type (Table 1).
Overall characteristics of the sample: patients with thin primary cutaneous melanomas and metastatic sentinel lymph node involvement (2003-2015, Laboratory of Pathology, Santa Casa de Porto Alegre, Brazil); n= 137
| SLN involvement | |||
|---|---|---|---|
| Variables | Yes (n=10; 7.3%) | No (n=127; 92.7%) | P* |
| Age (years) | 52.1 ± 12.5 | 53.9 ± 13.4 | 0.682 |
| Sex – n (%) | - | - | 0.104 |
| Male | 7 (70.0) | 53 (41.7) | - |
| Female | 3 (30.0) | 74 (58.3) | - |
| Location – n (%) | 0.533 | ||
| Lower limbs | 0 (0.0) | 22 (17.3) | - |
| Trunk | 6 (60.0) | 61 (48.0) | - |
| Head and neck | 2 (20.0) | 10 (7.9) | - |
| Upper limbs | 2 (20.0) | 27 (21.3) | - |
| Vulva | 0 (0.0) | 1 (0.8) | - |
| Acral | 0 (0.0) | 6 (4.7) | - |
| Type – n (%) | 0.567 | ||
| Superficial spreading | 9 (90.0) | 113 (89.0) | - |
| Lentigo | 0 (0.0) | 8 (6.3) | - |
| Nodular | 1 (10.0) | 6 (4.7) | - |
| Clark – n (%) | 0.005 | ||
| I | 0 (0.0) | 1 (0.8) | - |
| II | 1 (10.0) | 44 (34.6) | - |
| III | 5 (50.0) | 52 (40.9) | - |
| IV | 3 (30.0) | 30 (23.6) | - |
| V | 1 (10.0) | 0 (0.0) | - |
| Breslow – mean ± SD | 0.68 ± 0.26 | 0.65 ± 0.24 | 0.635 |
| <0,76 | 5 (50.0) | 76 (59.8) | 0.740 |
| ≥0,76 | 5 (50.0) | 51 (40.2) | - |
| Intensity of TIL – n (%) | 0.080 | ||
| Negative | 1 (10.0) | 3 (2.4) | - |
| Few | 6 (60.0) | 39 (30.7) | - |
| Moderate | 3 (30.0) | 63 (49.6) | - |
| Marked | 0 (0.0) | 22 (17.3) | - |
| Location TIL – n (%) | 0.512 | ||
| Peritumoral | 6/9 (66.7) | 67/124 (54.0) | - |
| Peritumoral and intratumoral | 3/9 (33.3) | 57/124 (46.0) | - |
| Regression – n (%) | 0.096 | ||
| Positive | 3 (30.0) | 76 (59.8) | - |
| Negative | 7 (70.0) | 51 (40.2) | - |
| Ulceration – n (%) | 0.141 | ||
| Positive | 1 (10.0) | 1 (0.8) | - |
| Negative | 9 (90.0) | 126 (99.2) | - |
| Satellitosis – n (%) | 0.505 | ||
| Positive | 1 (10.0) | 8 (6.3) | - |
| Negative | 9 (90.0) | 119 (93.7) | - |
| Mitoses – n (%) | 0.686 | ||
| Positive | 9 (90.0) | 101 (79.5) | - |
| Negative | 1 (10.0) | 26 (20.5) | - |
One hundred and thirty-seven cases of TPCM that had protocol indication for the procedure were submitted to SNLB (presence of ulceration, mitosis, thickness more than 0.75mm, regression), among which 10 (7.3%) had metastatic lymph node involvement. After multivariate regression analysis, only two factors had statistical significance. The group that had moderate and marked TIL showed a reduction in the risk of sentinel lymph node involvement, with OR=0.2 (95% CI: 0.02–0.72 and p=0.014), and those with ulceration had a 12.8-fold higher risk of lymph node metastatization: OR=12.8 (95% CI: 2.77–59.4 and p=0.001); these findings are shown in tables 1 and 2.
Multivariate analysis* to evaluate independent factors associated to sentinel lymph node involvement in thin primary cutaneous melanomas (2003-2015, Laboratory of Pathology, Santa Casa de Porto Alegre, Brazil); n= 137
| Variables | Odds Ratio (95% CI) |
|---|---|
| Sex | |
| Male | 3.10 (0.75 – 12.9) |
| Female | 1.0 |
| Clark | |
| I/II | 1.0 |
| III | 4.43 (0.40 – 49.7) |
| IV/V | 4.11 (0.28 – 60.4) |
| Intensity of TIL | |
| Negative/Few | 1.0 |
| Moderate/Marked | 0.20 (0.05 – 0.72) |
| Regression | |
| Positive | 0.63 (0.17 – 2.39) |
| Negative | 1.0 |
| Ulceration | |
| Positive | 12.8 (2.77 – 59.4) |
| Negative | 1.00 |
In 4 cases, Breslow was less than 0.76 mm. One of the cases of positive sentinel lymph node classified as low risk (Breslow 0.38, one mitosis per field, moderate TIL, presence of regression, negative clinical examination for lymph node enlargement, absent ulceration and satellitosis) had six metastatic lymph nodes out of six resected and resection of 25 more lymph nodes with no malignancy, constituting an exception case.
The ROC curve demonstrated a non-statistically significant (p=0.282) tendency that thickness ≥0.79mm would increase the chance of sentinel lymph node positivity.
The lowest Breslow thickness associated to positive sentinel lymph node was 0.31mm. In 4 cases (40%), the identification of metastasis in sentinel lymph node was only possible with immunohistochemistry. Among the patients who were submitted to lymph node resection, there was metastatization to other lymph nodes in 50% of the lymph nodes detected with immunohistochemistry. For the sentinel lymph nodes positive on hematoxylin & eosin, lymphadenectomy found other implants in 66% of cases. The mean number of resected lymph nodes was 27.5.
DiscussionIn the present study, the presence of moderate/marked TIL was a protection factor for the presence of metastatic lymph node. Patients with moderate/marked TIL in the primary melanoma lesion had a five-fold lower risk for the presence of one positive lymph node when compared to individuals with absent or few TIL in the lesion.
An increasing number of studies show that the individual immune response related to TIL can yield consistent results regarding improved survival, defining TIL as a prognostic factor.18-22 However, the use of this information in the early management of TPCM is limited. In the studies by Burton et al.23 and Duprat et al.24, the active presence of TIL demonstrated sentinel lymph node protection in melanomas thicker than 1mm. In the studies by Fortes et al.19, Mandala et al.25 and Taylor et al.26, TPCM were included with the thicker melanomas and the group demonstrated a higher risk for SLNB positivity when TIL was absent or few/inactive. Azimi presented similar results to those authors but the analysis was of all primary cutaneous melanoma and the thin group was limited to thickness between 0.75 to 1.0mm.18
The presence of mitoses did not constitute risk for sentinel lymph node positivity (p=0.686), contrary to what was observed by Kesmodel et al.27
It is extremely important to consider that local control of the disease in patients with lymph node involvement can improve disease- and recurrence-free survival when done early (SNLB followed by lymphadenectomy), with better local control of the disease (avoiding lymph node tumor masses, many times associated to ulceration, infection and bleeding, situations of difficult clinical management and great suffering for the patient), despite not improving overall.28-35
As limitation of the study, the coherence with studies by Azimi et al.18 and Fortes et al.19 in the characterization of ulceration as a risk factor for sentinel lymph node should be evaluated carefully, because even by demonstrating a significantly high risk (12.8-fold), the number of cases of ulcerated melanomas was small in both groups.
In the present study with 137 TPCM cases, we observed that the risk for SLNB positivity is not negligible (7.3%) and regardless of other risk factors, absent or few TIL presents as a risk factor for sentinel lymph node positivity on TPCM. Thus, it is important to take TIL into consideration in the group of information that will define which TPCM can benefit from SLNB.
ConclusionThe evaluation of the presence and intensity of TIL in invasive TPCM patients can help in the decision-making to perform SLNB.
Financial support: None.
Conflict of interest: None.